Castor EDC 2021.5 Release notes
Table of Contents
Major release 2021.5.0.0 Release date 11th October 2021
Added features and / or functionalities
Record Status mechanism
Tracking records throughout the study lifecycle is critical. For most users, whether processing individual records, or summarized views of study progress, rely on this information for critical user-flows. We’re introducing a first version of this new feature, allowing users to easily define and manage records statuses. These can then be assigned to single or multiple records and can be viewed in the records overview.
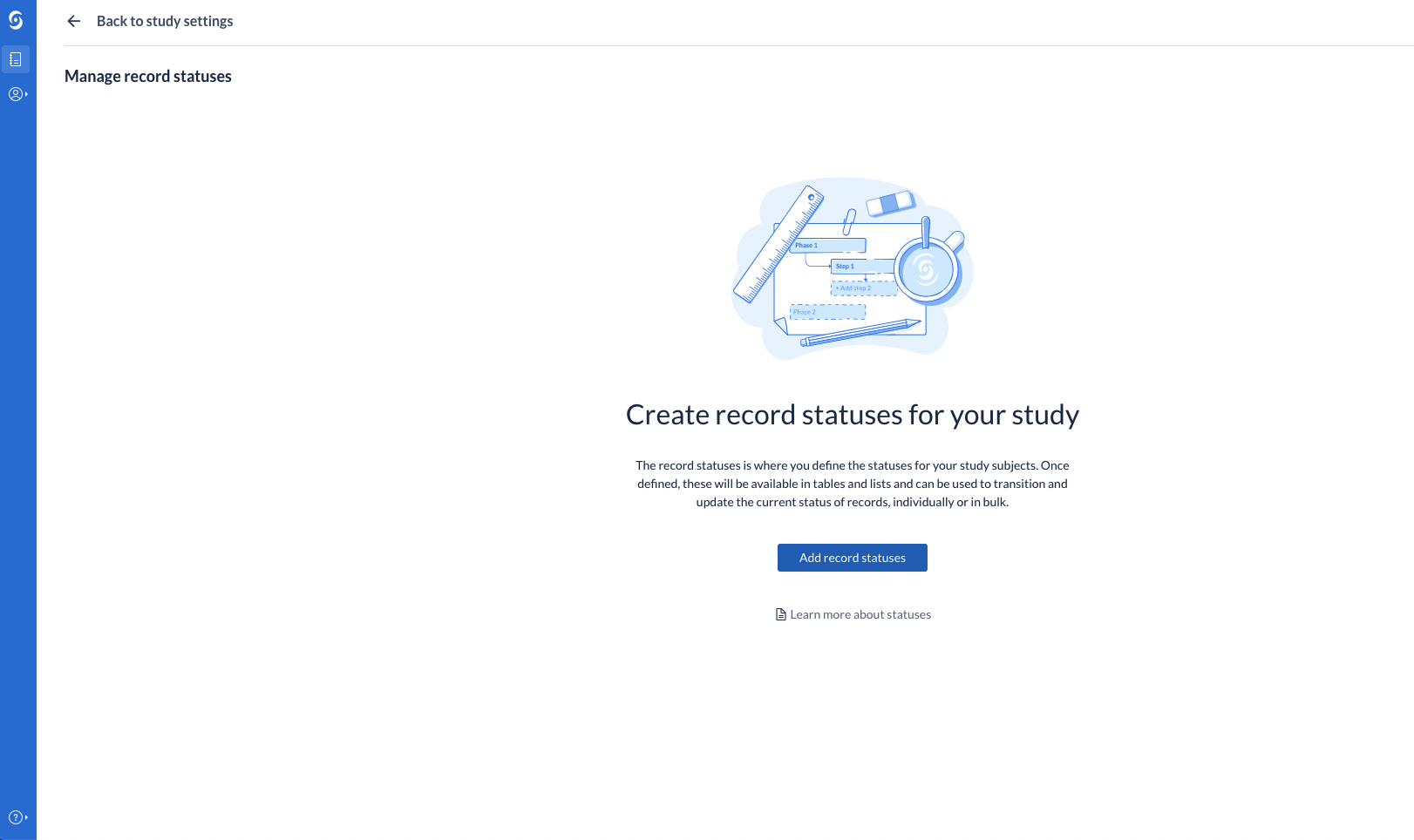
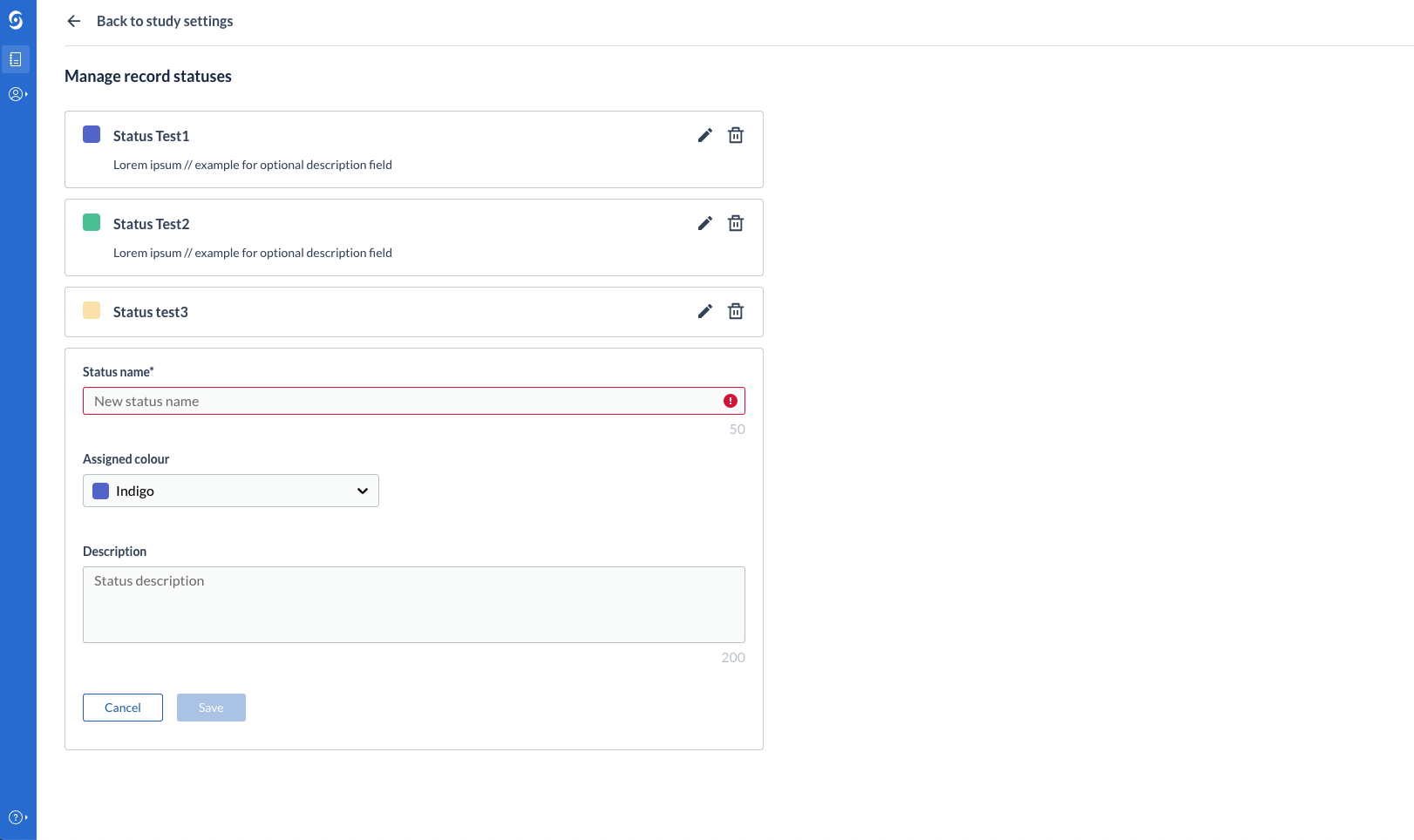
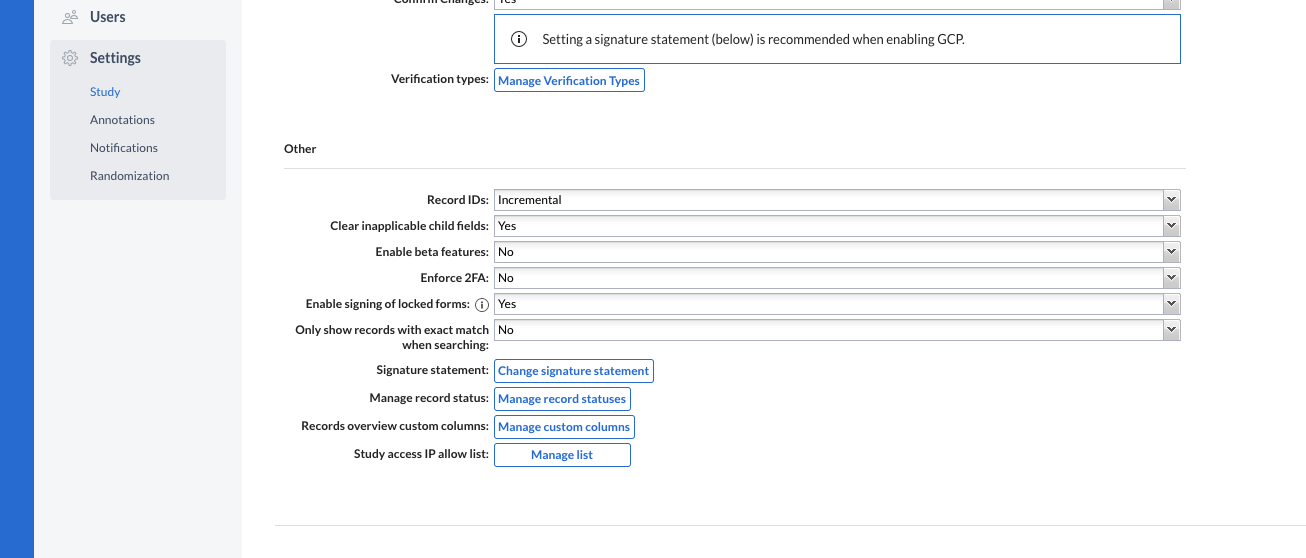
Users can create, update and delete record statuses from Study Settings. Manage Settings rights are required to perform this operation. Additionally, a color can be assigned to each created status, for increased visibility on the records overview and in data entry.
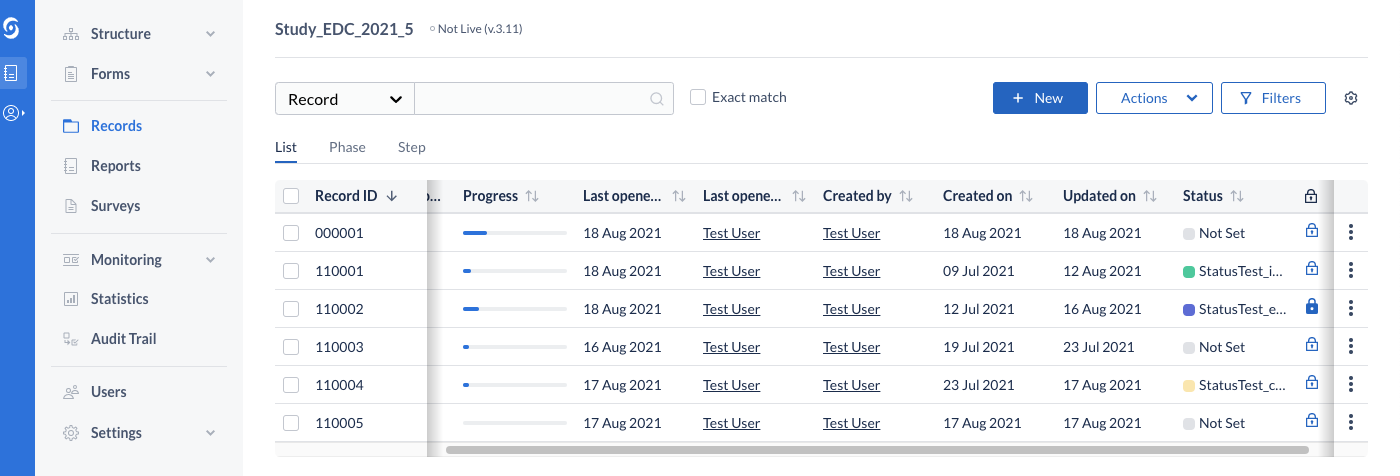
Users can see the current status of records on the records overview table, in the column labeled ‘Status’, as well directly in the data entry view. In order to access this information, users require View Institute rights.
On records overview, users can filter by results by record status. If no record statuses have been defined in Study Settings, an informative text is shown in the filters panel next to the new filter criteria.
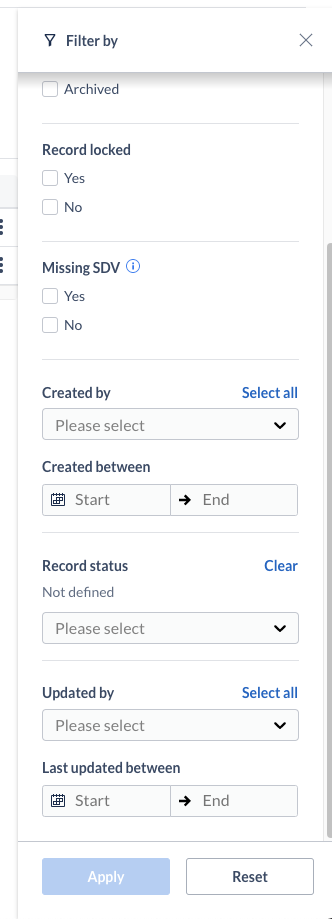
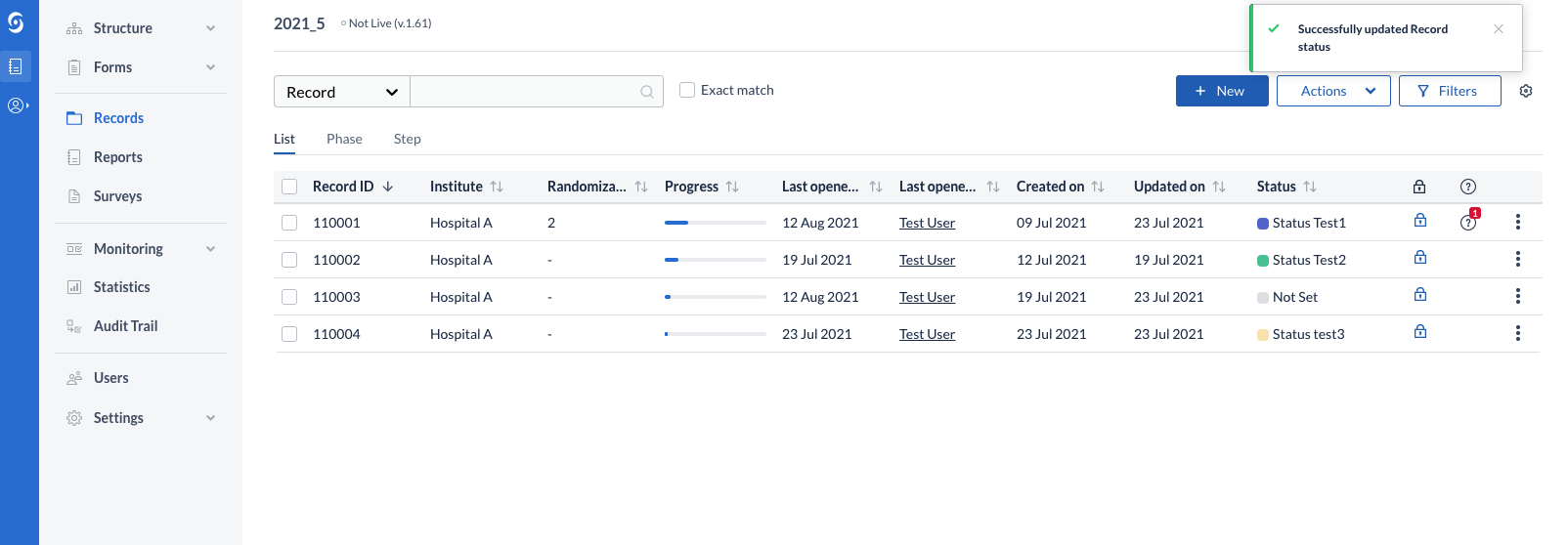
Users can change the current status of a record (transition a record from one status to another). This can take place from the records overview table, as well as from data entry (single record). If no statuses have been created on the study, the user is informed of that and can directly jump to create a new status in the Study Settings.
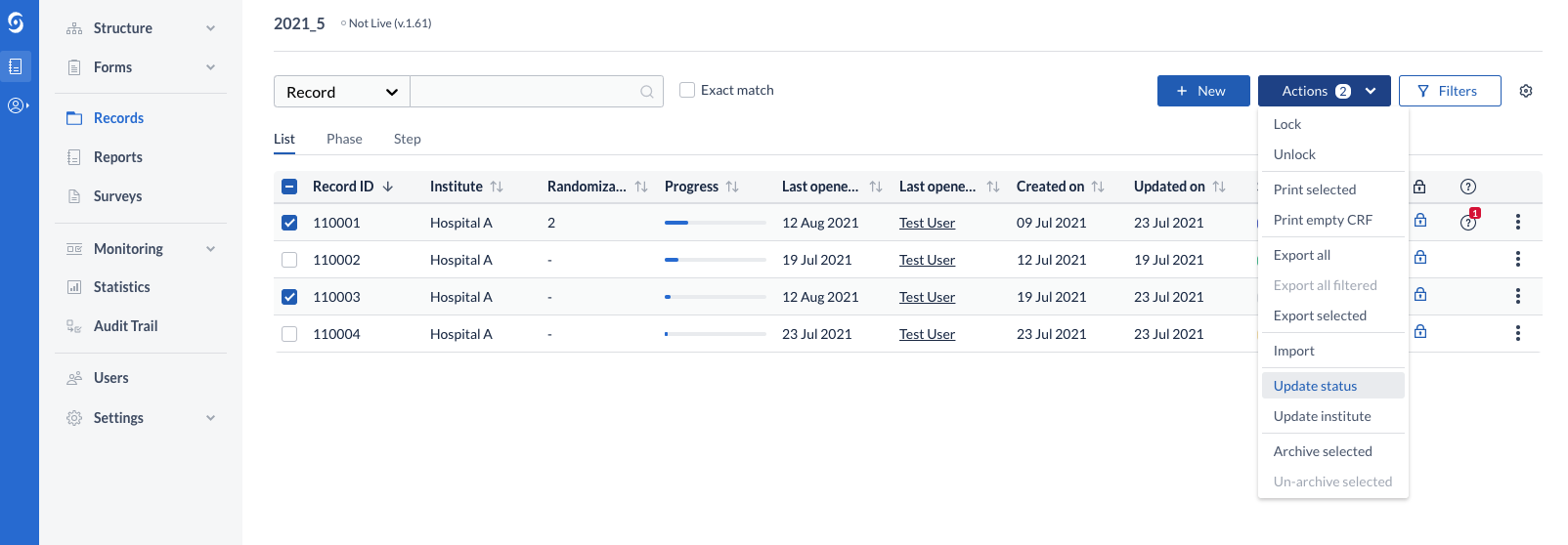
On records overview, the status can be changed for a single record from the available kebab menu or for multiple records at once, from the “Actions” menu.
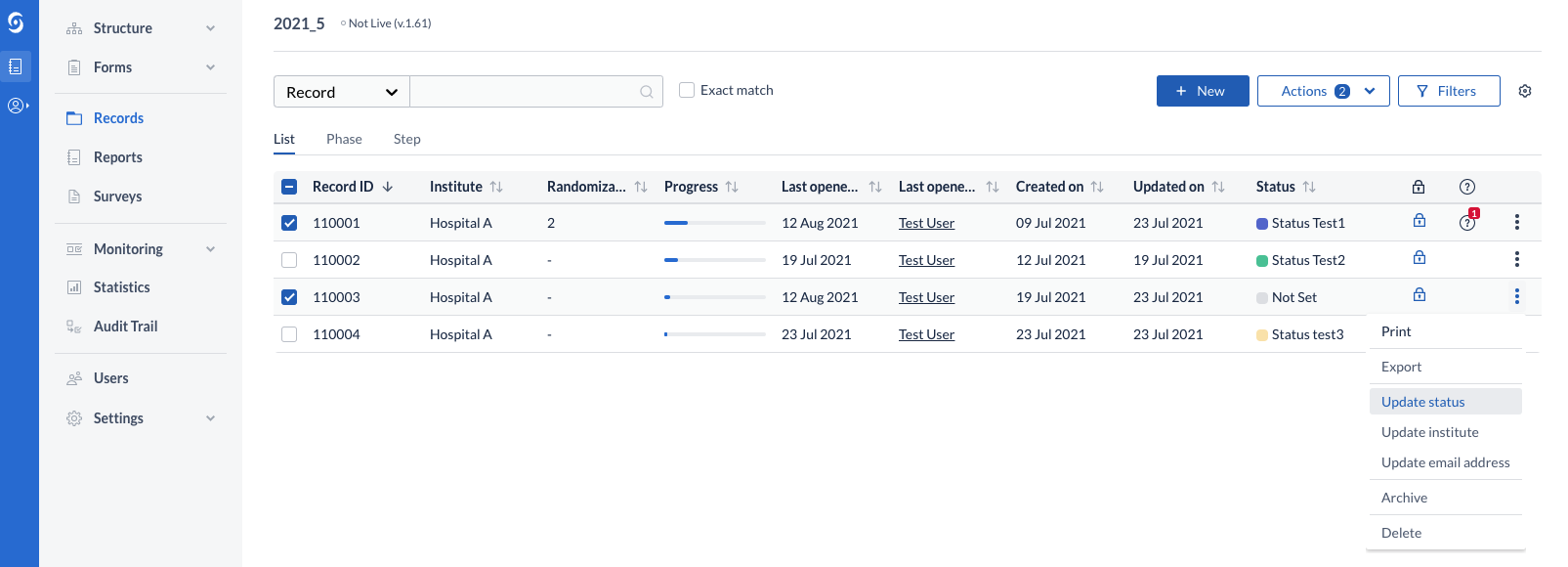
Within data entry, the status can be updated by directly clicking on the current status in the navigation bar or by changing the selected option in the status dropdown. In order to perform this operation, users require View Institute rights.
NOTE: the dropdown is a quick and direct way to change the status on the fly, while on data entry and therefore will skip the option to add a reason for the record status update. If a reason needs to be added, please use one of the other three (3) ways to change this: In data entry, from the left-side navigation bar or in records overview, from the 3-dot kebab and the Actions menus.
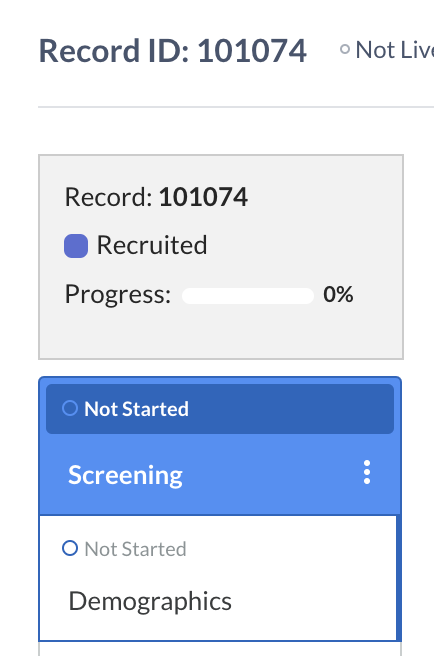
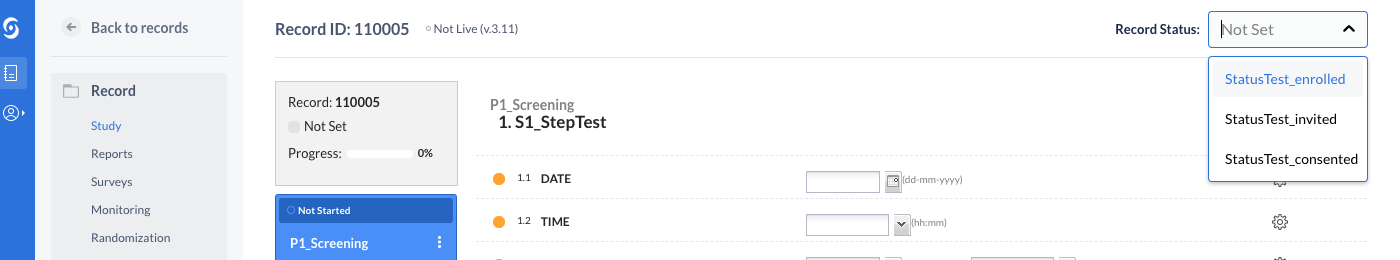
Upon creating a new record, either from the user interface or via the import, the status is set to a default value labeled “Not set”.
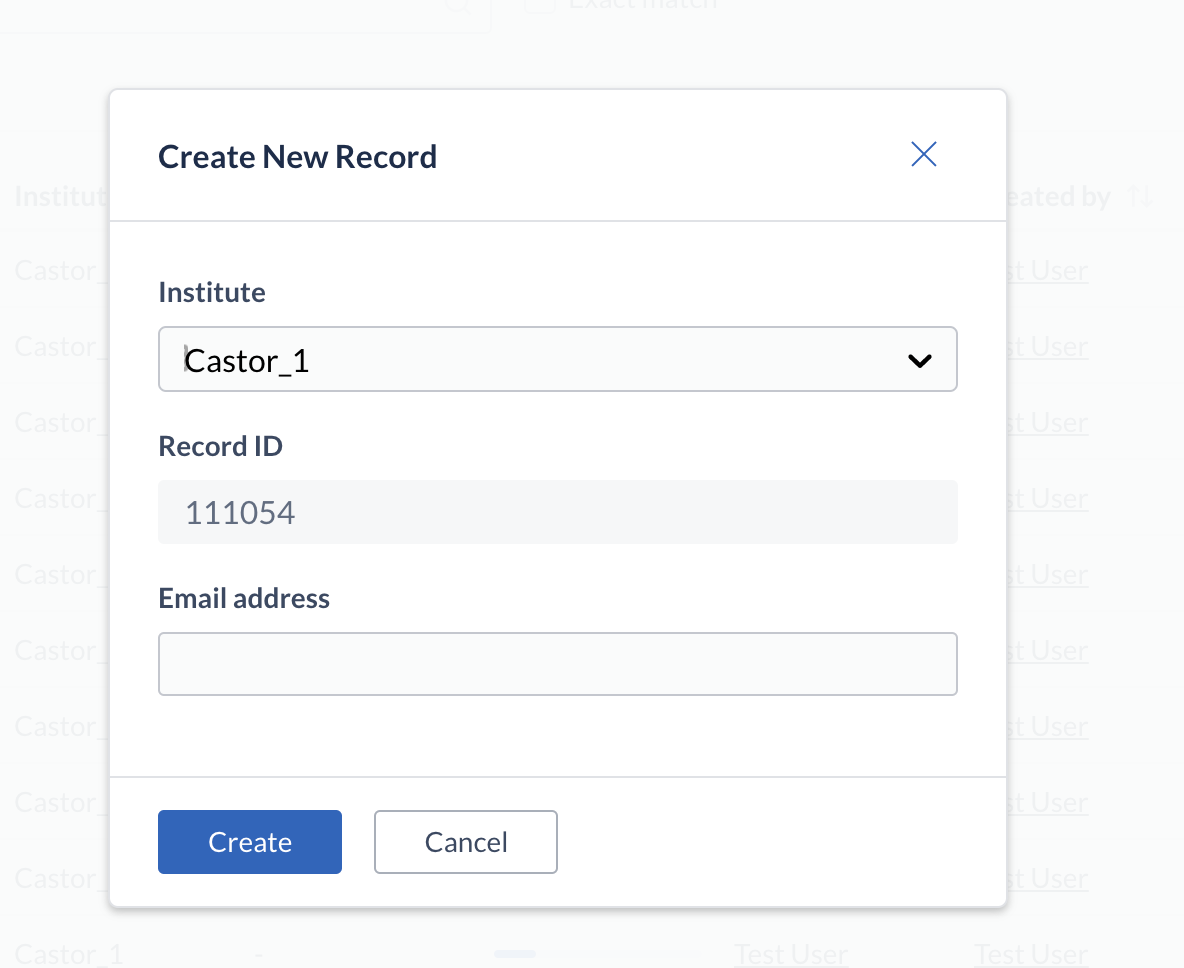
Option to add an email address from the record creation modal
Castor EDC users with permissions to create records can now opt to directly enter the record's email address and associate it with that record upon the creation of the records via the user interface.
New Audit trail event for Record status update
Under the Code of Federal Regulations- Part 11, specific and independent Audit trail events must be created when updating a record. With the introduction of record statuses in EDC, a new study level audit trail event has been included. Every time the status of a record is updated, a new ‘Record status updated' event is logged in the study.
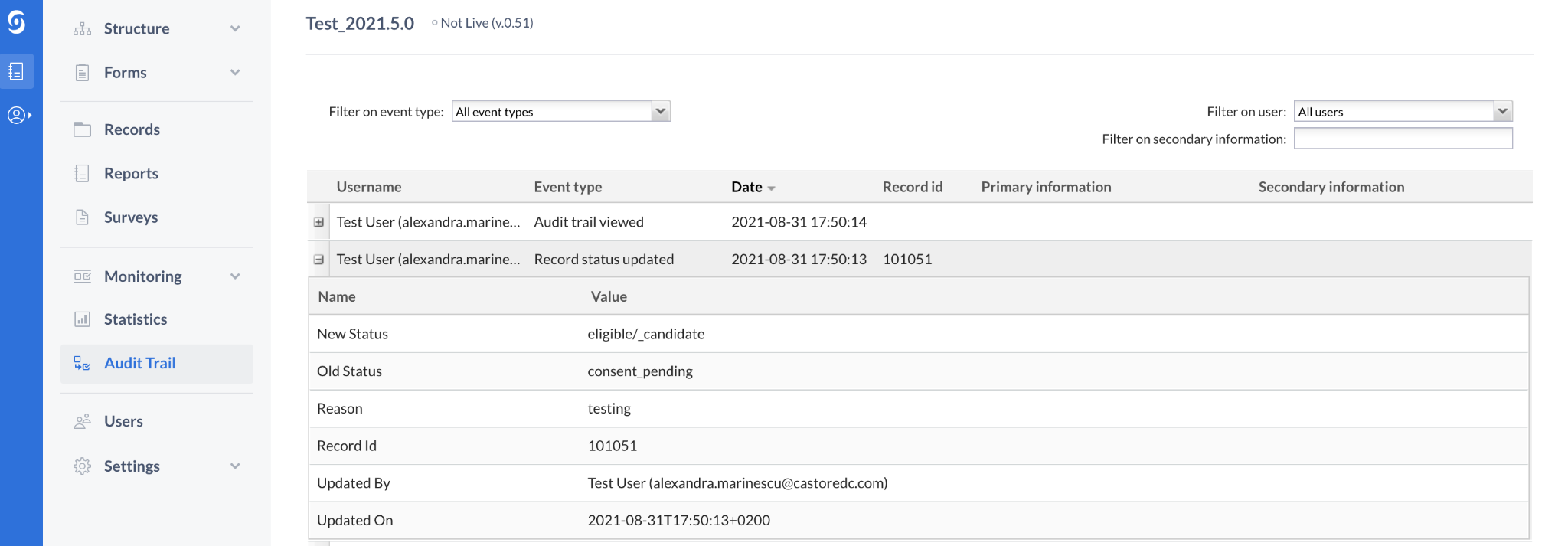
Additional Audit trail event details for Survey package start and end times
The time a survey package was started and completed (or received if completion time not available) will now be displayed within the audit trail, enhancing clinician visibility of participants’ survey compliance.
Additional insights on the Monitoring tab
Two new counter bubbles have added to the ‘Actions’ and ‘Filters’ of global Queries overview buttons to allow users to better understand how many filters have been applied to the current table view, as well as how many table rows have been selected, regardless if these are visible or hidden due to concomitantly applied filter criteria.
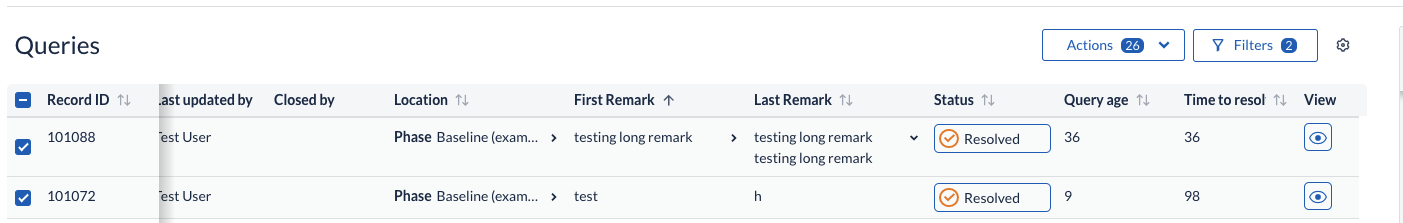
A cogwheel menu has been added to the Queries overview to allow users to configure at any point which columns to have displayed in the table listing.
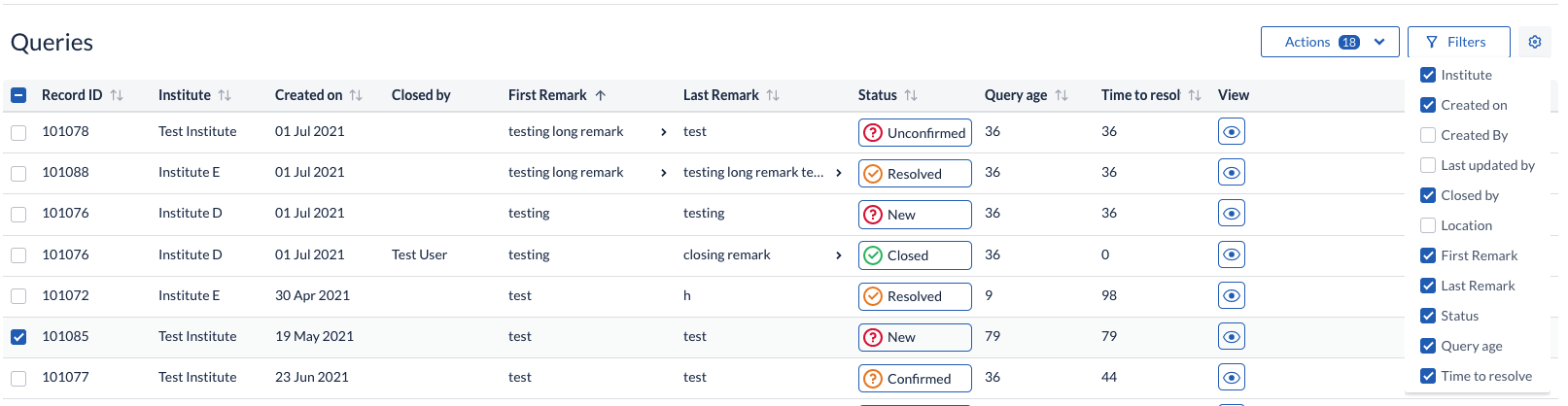
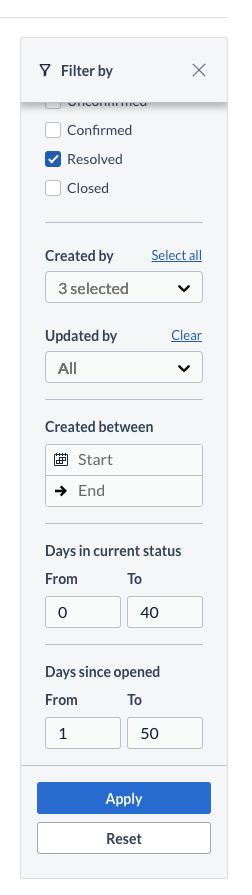
New filter criteria are now available on the Queries, Validations and Verifications filter panels, allowing user to reduce the visible
- Query listings by filtering on: Created by, Update by, as well as Query age (days since a query has been first opened and days since a query has been in the current status).
- Validations listing by filtering on: date range ('Triggered between')
- Verifications listing by filtering on: date range ('Dropped between')
The view title of the global Verifications has been updated to better showcase the information displayed in the table.
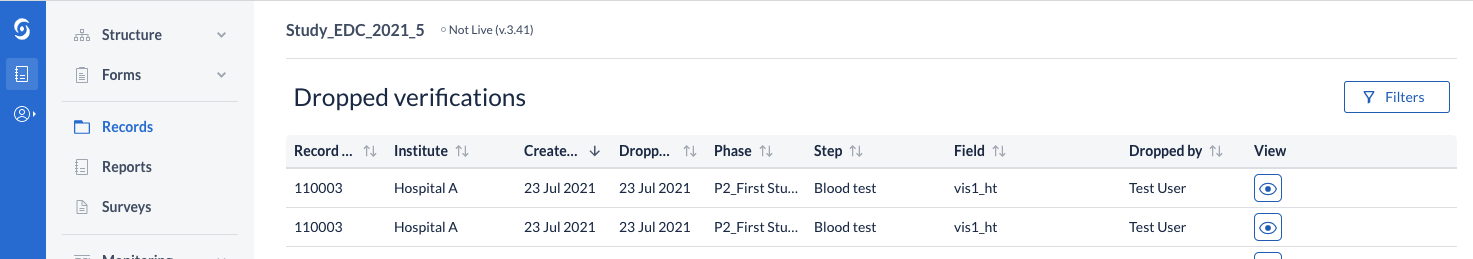
Open Survey Link
We provide new ways to invite participants into surveys. With this feature users can create a link to the survey and distribute it independently of the built-in email invitations; for example in the social media groups. Each participant filling the survey effects in a new record creation.
.png)
This feature currently is available upon customer request. To enable it please contact Castor support via support@castoredc.com and provide the study title for which this feature should be enabled.
Variables in link fields are available
In a new type of field - ‘Link’ - users can create a link to an external webpage that is generated in the same way Calculation and Summary fields are generated, using variables. With this approach users can create deep-links to external resources like PACS, HIS or other systems. Usage of a custom link requires a link template. The active link will be created automatically. As an additional safeguard the warning message will appear informing the user about leaving the CastorEDC.
.png)
.png)
.png)
Randomization date available as a calculation field variable
The calculation field variable list has been expanded to include the randomization date.
.png)
.png)
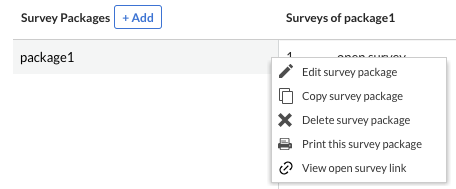
Flexible date format at institute level for data entry
As a first step towards allowing more flexibility in choosing the desired date and time format in EDC, we’re first introducing the possibility to define, per each available study institute, the preferred date format to be used and displayed within data entry.
In the ‘Manage Institutes’ modal, users can see and choose to update the selected date format. A list of eight (8) different formats is available, from which users can select one for each institute.
For all new studies, the default date format will be the standard ISO format, while for running studies the default will remain with the previous format that was available before EDC version 2021.5.
Depending on the institute date format, the view in data entry for each record will reflect that on all relevant field types: Date, Date and Time, Date and Number.
NOTE: Additional functionalities on this topic will follow in upcoming releases!
New text field linked to billing codes
An additional text field labeled "Cost center (department) no." has been added and linked to the billing code; Once a valid billing code is entered, the new fields will be displayed below, allowing users to add additional information regarding specific project numbers if needed.
Removed features and / or functionalities
When locking SPI in the API, the required Edit right has been removed
We have now removed the support for ‘Edit’ right when locking a Survey Package Instance from the API. Locking via the API is now possible with ‘Lock’ right, aligned with the existing behavior to lock a Survey Package Instance via the user interface.
"Next phase" column hidden from records overview
We have temporarily hidden this column from the Records overview, as this column did now display any information ( empty for all records). It will be reintroduced later on, when all needed implementation is in place so that users can benefit from the “Next phase” column data.
Deprecated Audit trail events removed Study Audit trail dropdown
Some options in the filter dropdown have not been in use (empty or duplicated) and are now not visible any longer, to allow the system user to filter the audit trail log unambiguously.
Improved and extended features and / or functionalities
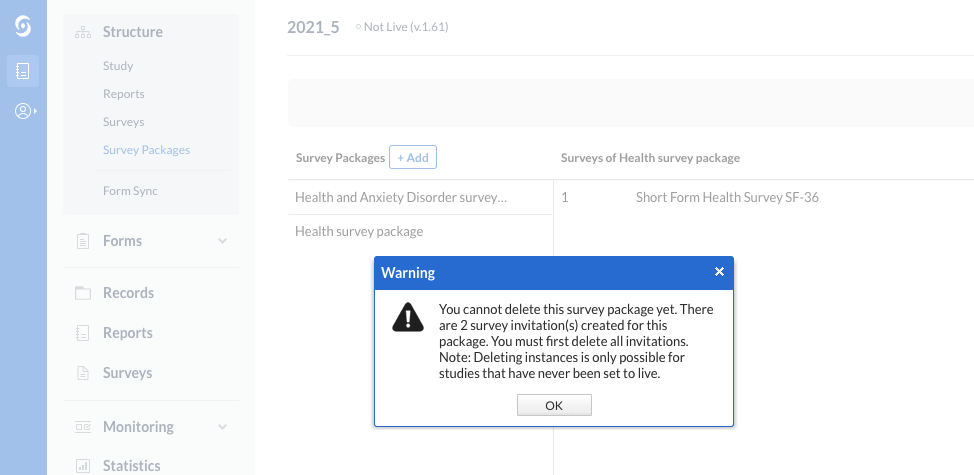
Updated criteria and warning message when deleting survey packages or reports
When there are active or archived instances created for a survey package or for a report, an updated warning message is displayed to the user when attempting to delete the element.
Randomization supports now more than NINE randomization groups
Some new studies require more than nine randomization groups. We updated the algorithm to support up to 35 randomization groups.
User management API endpoint
We extend the /study/{studyId}/users endpoint with access to roles and permissions data. Additionally, the new POST method allows the creation and invitation of users to study.
Roles management API endpoint
This new /study/{studyId}/role endpoint allows headless roles management. By calling this endpoint, the user can create new roles with customised set of rights. The endpoint also provides full information about all the roles created in the study.
Institutes management API endpoint
We extended the /study/{studyId}/institute endpoint capabilities. Now it allows the creation of new institutes.
Audit trail API endpoint
The audit trail is a crucial part of study documentation. We facilitate access to it in /study/{study_id}/audit-trail/ endpoint. The authorised users can retrieve the selected time range of the audit trail events or filter by event types and user ID.
Due to changes in the audit trail architecture, Queries and Comments created before Aug 01 2020 are not available via this endpoint. Nevertheless, these are always accessible via the Audit Trail user interface.
NOTE: When accessing the Audit trail, we have improved the readability of creation dates when the audit trail events are displayed in the extended event view. Date and time are displayed in ISO8601 format now.
Verifications endpoint
Along with the audit trial access we add the new /study/{study_id}/verifications endpoint to retrieve SDV and custom verifications themselves. It shows the current state of verifications in the selected study and allows filtering by records, time frame and verification types.
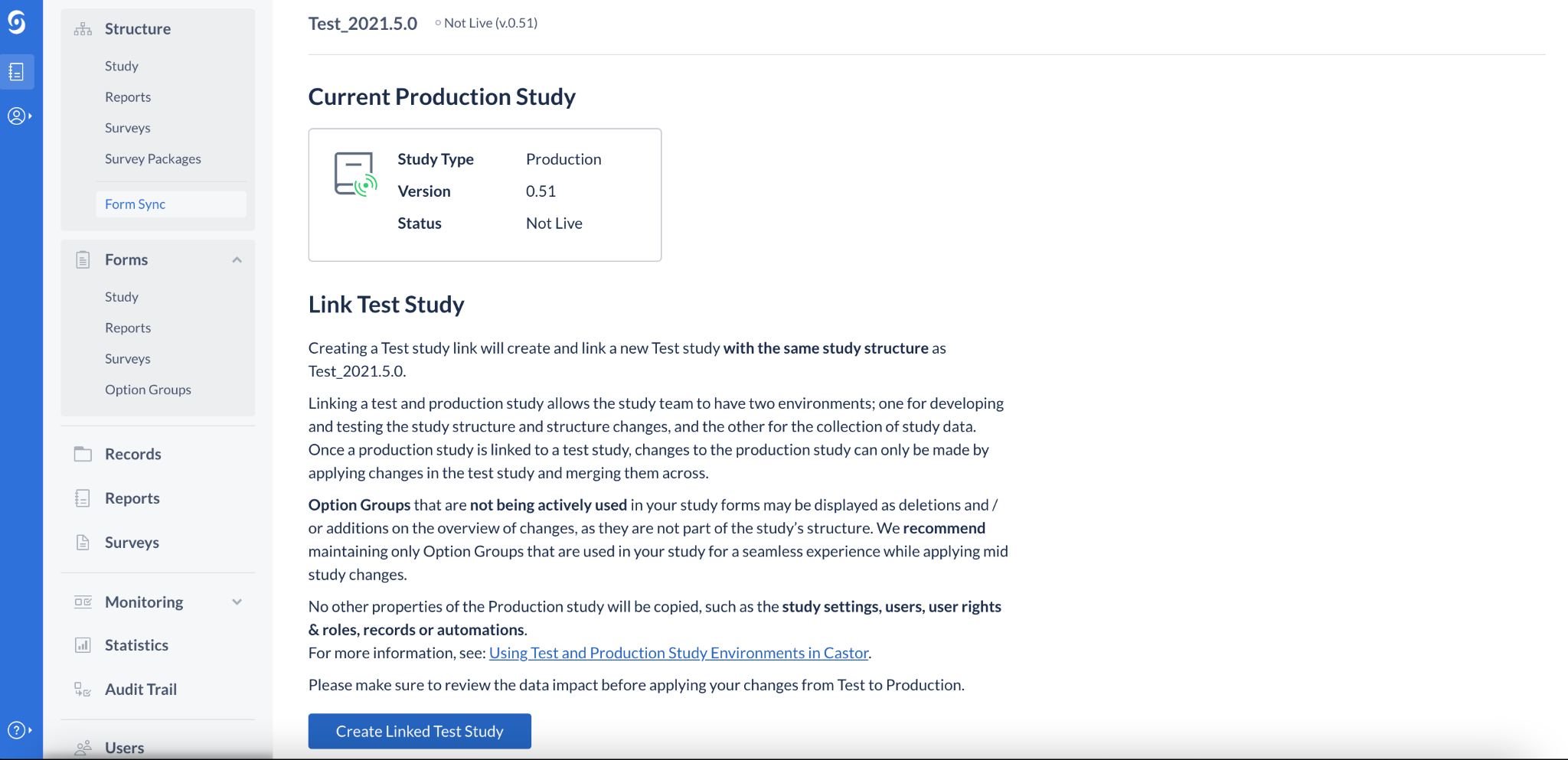
Updated static text on Form Sync view
The static text shown on the initial view of Form Sync has been updated to better inform users of how Option Groups that are not being used in the study are identified during a mid-study update process by the EDC system.
Mouse focus extended on the first field of modals
To better address the needs of users with limited movement and/or physical disabilities, we have extended the default mouse focus on more modal dialog elements. The modals affected by this can be found on Records, User settings, My studies and Monitoring views and all field types that can be focused by default now have this feature included.
Query export file row visibility
Export file used to contain multiple lines for each remark added for the same query, instead of 1 single line for the last remark. This improvement has been implemented looking ahead to future product improvement to allow multiple queries per field. In this upcoming scenario, the export files would be confusing to interpret by users.
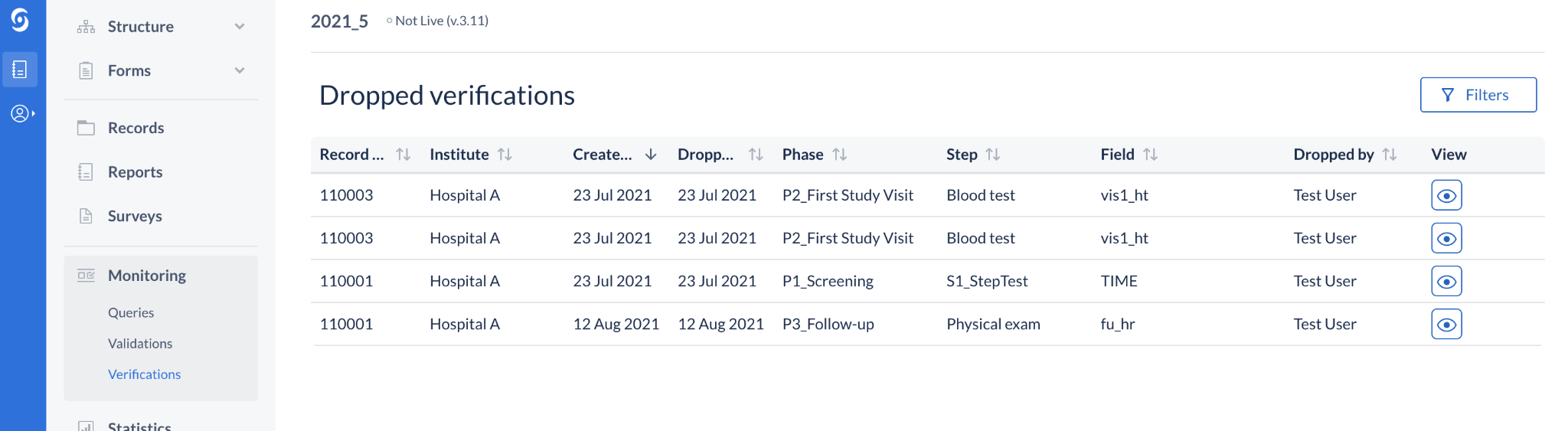
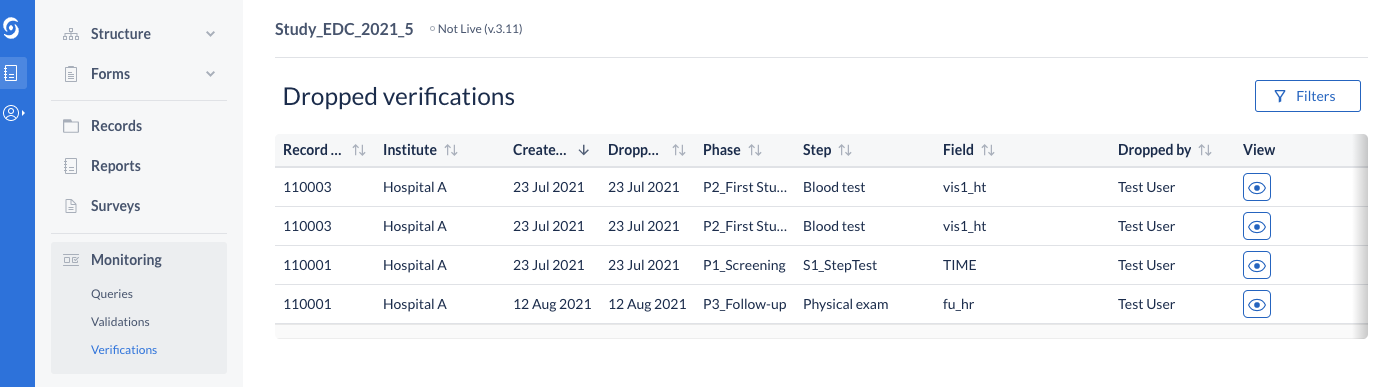
Updated table title for Verifications
The data listings for verifications in the Monitoring tab is showing a list of global, study - level dropped verifications. The title on the view has been updated to reflect that.
Updated information on the User Registration Form
When a new user lands on the EDC registration form via an email invite, the marketing opt-in checkbox was not visible. This has been included in the ‘Complete account information’ form for consistency with the Sign Up form.
Displaying Repeated measurement table in data entry
When a new report instance of the type "repeated measure" is added via a repeated measure field, but no data is entered in that report instance, the table view shown in data entry now displays the creation date of that instance (instead of an empty table).
Laying foundations for future features and known release limitations
Video calling with patients from EDC
As part of this release, we have deployed new functionality enabling clinicians to initiate video-conferencing calls with their participants directly from within the EDC.
This functionality is intended to be made available more widely as part of a future release, thus is not available for general use and will not be visible to end users until that time. To ensure no accidental access is provided to users, the feature is only available when the feature is both turned on at the study level by Castor and specific permissions are granted to users on said study.
Extended export capabilities for ODM compliance
We are working to provide our users with more options, more flexibility, for import / export actions so that Castor EDC can help the exchange of data and metadata between different systems. To achieve this goal, we have kicked off an initiative toward supporting the CDISC ODM format.
As a first milestone, CDISC ODM data and structure export functionality in its first version is now available for testing purposes and early feedback. As this is not going to be directly accessible to all users in the 2021.5.0 release, please reach out to our Support team at support@castoredc.com to request access to it.
.png)
Display records and surveys without data while accessing them via API
Including Reports and Surveys without data while accessing them via API /data/export endpoint
We'd like to inform you that from the next release - 2021.6 - we plan to append additional data to the following endpoints: /study/{study_id}/export/data. Currently empty records are already exported, with the upcoming release we will also add entries for empty Report Instances (RIs) and empty Survey Package Instances (SPIs). Currently for either of these at least on datapoint needs to be present in order for the RIs and SPIs to be listed. In order to do research on missing data and/or subjects that are lost to follow-up, we will be including these empty RIs and SPIs by default. A query parameter will be added to exclude the empty instances if needed.
System defects fixes
- Monitoring:
- Range filters did not included border values
- Filtering Queries in the Monitoring tab in a Record view fix
- Archived reports information was still shown in Monitoring - Validations list
- Verifications for archived/deleted reports were not displayed
- Form Sync: Newline inconsistency fix
- Records:
- Popup was displayed instead of toast message when creating > 10 records for not live study
- Add record button was missing in Records tab when the study did not have form fields
- Reports:
- Summary field value was not displayed when printing Report Instances
- count inconsistency fix for reports hidden for user roles
- Surveys:
- Outro text was displayed as a blank page for each survey
- when pressing 'finish survey', the survey window was not closing (in Incognito mode)
- Export:
- 0 was displayed instead of NULL/empty for empty fields in exported files
- fix in archived entities during export of queries
- User settings: First Name of user could not be changed
- Print:
- Grid Column Headers were not included in printed versions of Blank Forms
- Option value were displayed instead of label of that Option in print view
- "Print options" dialog was not displayed on the Structure tab
- Registration page: Password length validation was not triggering on frontend side if spaces were added
- New Study: Country list was not sorted alphabetically
- API: Query API documentation fix
- Lock / User rights: Fix so that users can lock a survey with Survey rights
- Data entry: Empty window was displayed when the loaded step/phase was configured to be hidden for the user's role
- Audit Trail / Form builder: 'Created field' event was logged incorrectly
2021.5.1 - 12 October 2021
- Fixed a defect where an Error was displayed if a user without Manage Settings rights tried opening Record Overview
- On Australian server, users can freely turn the Monitoring feature on and off
2021.5.2 - 14 October 2021
Fixed a defect where a message was shown "Please wait, we are building the form..." for records containing dates in the incorrect format (for example, D-MM-YYYY)
2021.5.3 - 21 October 2021
- Fixed a defect where fields without a variable name rendered an error in SPSS
- Increased FormSync time threshold
2021.5.4 - 26 October 2021
- Added prefix to deleted record IDs
- Fixed a defect where field level audit trail kept old entries when a record ID was re-used
- Fixed defect for CDISC export of data containing certain special characters
- Fixed a defect related to saving values for dropdown fields inside grid field
2021.5.5 - 5 November 2021
- Fixed defect regarding Reports. For studies with a large number of institutes and/ or blinded reports, the report instances failed to load due to performance issues.
- Reversed change regarding exporting checkboxes without inputted values, introduced in 2021.5.0;
We have now rolled back on a recent change, going back to having ‘0’ (no value inputted) or ‘1’ ( value inputted) in the export field for checkboxes. All other field types that have no data will be exported as empty. - Fixed defect regarding Tableau dashboards. An error was displayed to the user after navigating away from the tab.
- Minor change in API regarding survey packages enabled for Castor Connect.
- Image fields will be configurable in mobile survey packages (pending release 2021.2 of the Castor Connect).
2021.5.6 - 12 November 2021
- Fixed issue regarding steps hidden for user roles that were visible in isolated cases, where a second study user would log in soon after the previous user and would have had access to the last opened step.
- Fixed issues regarding the structure tree not being populated while attempting to export data.
- Fixed issues regarding error appearing when exporting the study structure with without any phases or steps.
- Amended the Statistics tab, with regards to:
- Default sorting on the ‘Country’ filter dropdown and column
- Restricting the selection of the ‘Institute’ list options, based on previous selection of a country
- Displaying the list of countries on the available lists (tables)
- Including more accurate information about the number of randomized records as per randomization groups and country.
- The granularity of the graphical representation for study inclusions can now show both weekly and monthly graphs.