Castor CDMS 2022.5.x.x Release notes
Major release 2022.5.0.0 - Release date 13th December 2022
Overview/Important Alerts
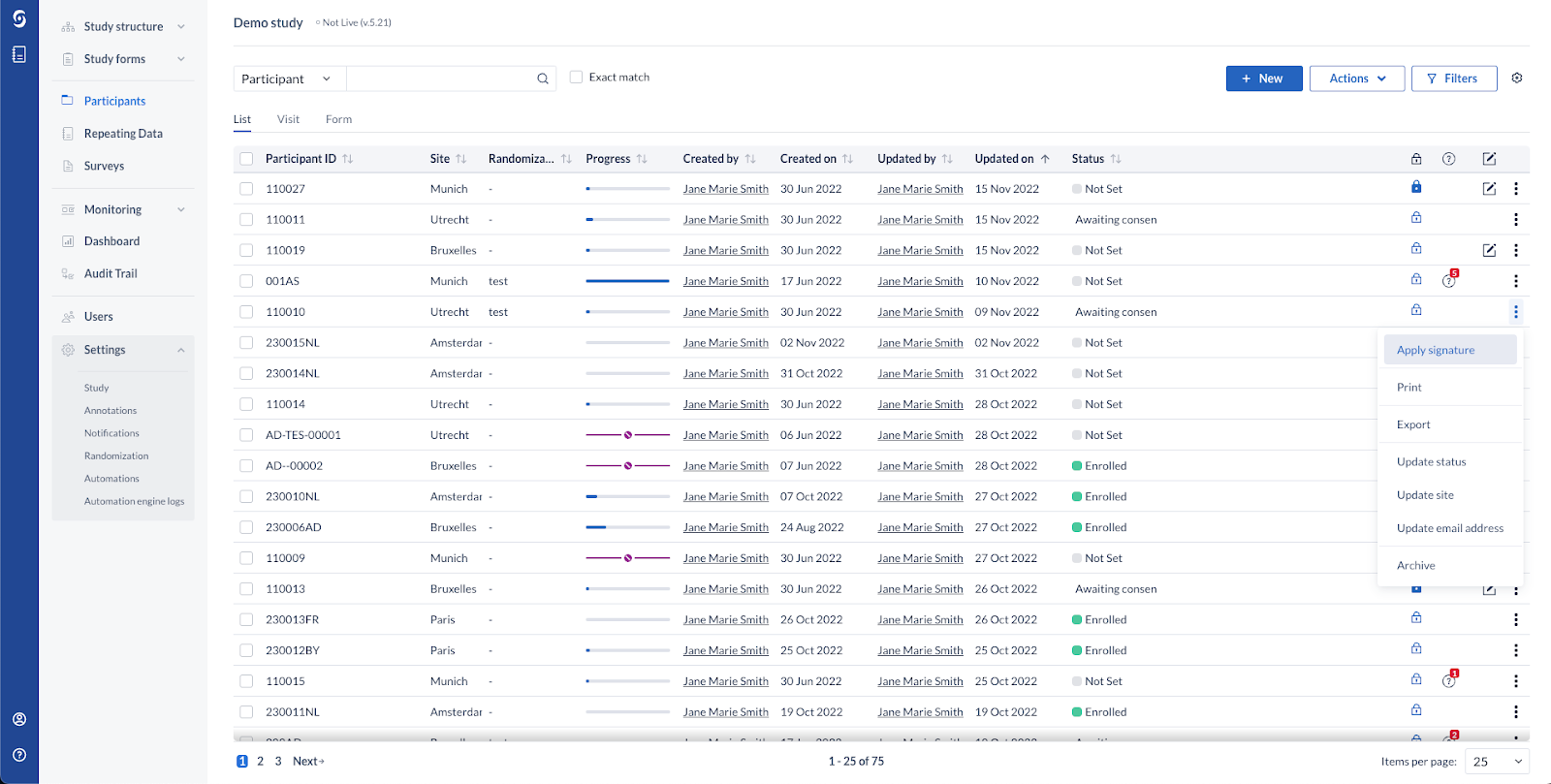
We are rolling out new system capabilities for electronic signing. From now on, you will have the ability to seamlessly sign all forms of a selected participant at once, directly from the ‘Participants overview’ page.
We encourage you to read further and explore in detail the entire scope of changes designed to ease the workload of repetitive actions.
New features and / or enhancement
Study Participants & electronic signatures
Users with ‘Sign’ right can now directly sign off all visible (according to the user's role) forms, visits, repeating data instances of a single participant from the overview.
All previously existing compliance requirements for the electronic signature have been kept and are now applied in batch to all affected forms when the action is performed by a logged in system user.
The option to sign / unsign a participant is found in the 3-dots menu on the ‘Participants’ page, available for users that have forms hidden for their assigned user role(s), but the blinded per role forms are skipped from being signed/unsigned.
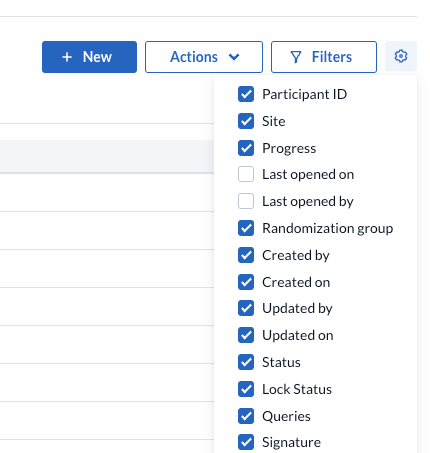
A new column is available on the Participants overview to indicate the signature status of a single participant record. By default this is selected, but it can be removed from the view by unchecking it from the cogwheel menu.
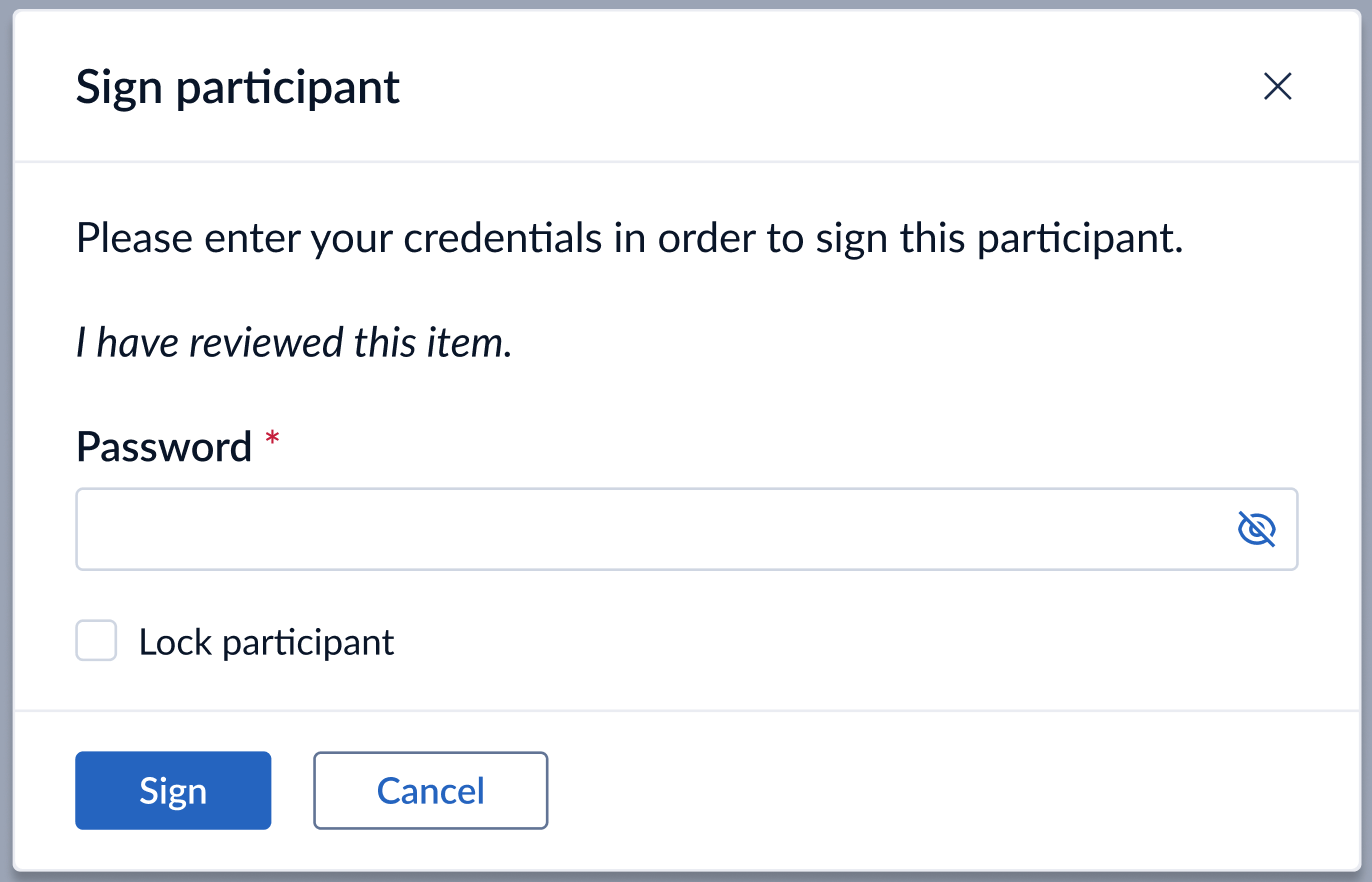
We kept the option to additionally lock a participant while signing all of its CRF forms. This is available in accordance with the assigned permissions ‘Sign’ and ‘ Lock’.
For studies without the option 'Enable signing of locked forms' enabled, when a participant is locked, the option to sign it is not shown in the menu.
The user will see an informative toast message regarding forms that have been skipped due to role blinding or pending queries. A dynamic success toast is shown every time a participant and all its forms are signed / unsigned.
When all available CRF elements of one participant get signed (one by one), the participant is marked as signed as well. Respectively, when the signature is dropped automatically for a child element, it cascades to its parent. In this logic, archived participants are taken into account as well, so that the signature status is correct and up to date when the participant is to be unarchived.
The signature is recalculated for a participant upon structural changes, data import and data entry (field dependency & active automations logic). For example, adding a new repeating data instance to a signed participant during import will cause the signature to be automatically removed from the participant level.
Study's audit trail
As now users have the ability to sign all forms of one participant, we're introducing new events in the audit trail: 'Participant signed' & 'Participants signature dropped'. These are logged in the system both when the event was triggered manually, by a logged in study user, as well as when it was triggered automatically by the system, as a direct result of another related action such as structure changes.
The events triggered automatically will be logged as having been done 'By system' and will not contain any additional information such as the signature statement. These details are only stored on the events that were initiated by actual study users.
Every time a logged in study user applies or removes a digital signature, specific and independent audit trail event(s) is (are) logged in the Audit trail for the action(s) performed by user.
Every time a digital signature is automatically applied or removed by the system due to another action or event that took place in the system, specific and independent audit trail event(s) is (are) logged in the Audit trail for the action(s) carried out by the system.
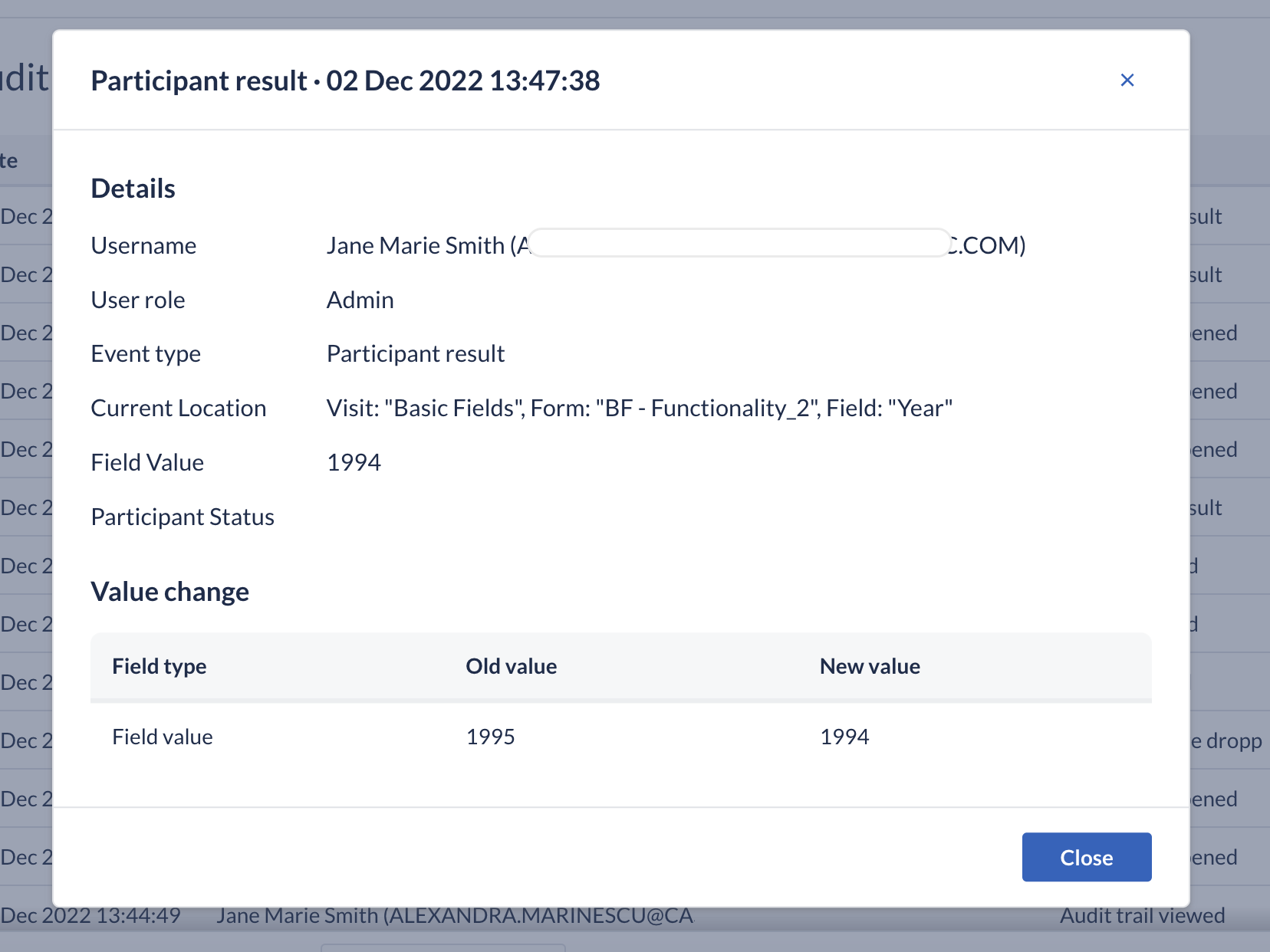
In this product release, we have also updated the ‘Field created’ and ‘Field updated’ events in the Audit trail, to additionally log information about dependency changes.
We are constantly working towards making reviews of the study data easier. In our improved Audit trail, while reviewing the event monitoring, the user can see old and new values. This gives a better overview of the trial progress and each data point's history.
Reporting capabilities: Dashboard, Statistics & Repeating data listings
We have expanded the drill down capabilities introduced in the previous major product update. Users can navigate from the 'Query status overview' table on the ‘Dashboard’ directly to the global queries listing, using the filters selected in the ‘Dashboard’ page, to easily reach more granular information.
The same capability was added to the 'Pending queries' graph on the Dashboard, as well as the query related tiles on the dashboard to the global query listing.
Entering & managing partial dates
- We now allow users to define date fields as allowing the entry of partial dates. This includes both a missing day, and a missing day and month.
- This will be reflected in configured web surveys, visit forms and repeating data forms (Castor Connect will be updated in an upcoming release to also allow this) and their printables.
- We also allow users, if a date field allows partial dates to be entered, to determine how those dates will be handled by dependencies and validations
- Ignore - partial values will be ignored by said validations and dependencies
- Match - a partial match will be used for the purposes of validations and dependencies
- Round up - partial dates will be rounded up to the latest calendar day for the purposes of validations and dependencies (e.g. March 2023 is treated as 31-03-2023)
- Round down - partial dates will be rounded down to the earliest calendar day for the purposes of validations and dependencies (e.g. March 2023 is treated as 01-03-2023)
- A custom component/method has been added to calculation fields to allow users to configure their own calculations dependent on partial dates.
- The migration of changes to whether a date field allows partial dates will be permitted using form sync.
- The importing and exporting of partial date values is permitted, as long as the relevant date field(s) in the form(s) are configured to allow partial dates - this includes all standard export formats offered by Castor.
Performance improvements
We've improved the performance of the opening of a study. Previously, when a study was opened, all study information was loaded (including information that was not displayed in the User Interface). To improve performance, we've ensured that only the information that needs to be displayed is loaded.
This includes the Study Structure and Study Settings (Study Settings, Annotations, Notifications).We have further improved the performance of user role checks (checks that hide certain parts of the eCRF for certain users) throughout the system. Our next release contains more of these performance improvements.
API
With this release, we introduce another Castor CDMS and eConsent improvement. Now, in the studies that have the eConsent participant flow enabled, Users can reach participant consent details directly from the participant view in CDMS. To enable this, we introduce a new resource: /api/study/{studyId}/participant/{participantId}/econsent
With this release, we start to enforce the rate limits for all of the endpoints at 600 calls per 10 minutes for CDMS API.
System defects fixes & minor changes
Forms
We have fixed a defect where it was possible to add a variable name to Repeated Measure fields, despite the fact that the variable name is not used in the system
We fixed a defect related to the importing for survey package senders for users that were not part of the study.
We have fixed a defect where structure items with duplicate names could be imported. Now you are presented with an error message stating which items in the import files are duplicated and should be renamed.
We have fixed a defect in the form structure import for partial visits where it was possible to import fields with the same variable name.
We have fixed a defect related to the importing of specific field types that are only enabled for certain studies.
We have fixed a defect related to displaying the Randomization group name in the Form Builder for fields that are dependent on a Randomization field.
We have updated the terminology used in the error message displayed when a visit is added with a name that already exists.
We have fixed a defect where it was possible to change the label and variable name of a field on a signed form, without confirming these changes.
We have aligned the validations (e.g., minimum and maximum input length) in the Add/Edit Field Modal with the validations in the previous design of the modal.
We have updated the size of the Calculation Template field in the Add/Edit Field modal.
Study participants overview
We have fixed a defect where the use of the date picker filter would cause an error on the participants overview.
We have fixed an issue that was hiding the lock icon from the overview of study participants for those users that did not also have lock permissions. This was now reintroduced as s disabled icon.
We've addressed an issue where the lock icon was unavailable after having archived a participant. This is not visible again on the participant's overview, in a disabled state.
We've fixed an issue on the participants overview, that temporarily showed archived participants post import even if the filter for archived was not selected.
Misc
We've addressed a minor issue regarding missing form validation in the user interface on the study name input field.
We've fixed an alignment issue of the archived study label.
We fixed the issue where only 10 survey instances returned for survey packages that contained more than ten instances.
We have addressed a defect that did not allow Survey Packages attached to ‘Add Survey Button’ field to update on the production study via FormSync.
We have updated the label for bulk SDV option in data entry for better readability and a more clear distinction between visit and repeating data forms.
Hotfix release 2022.5.0.1 - Release date 22nd December 2022
- We have fixed an issue related to signature drop following a new query being added to a previously signed form.
Maintenance release 2022.5.1.0 - Release date 29th December 2022
- We've addressed an issue where the 'delete' prefix was not being added to participant ID's for sone audit trail events.
- We've delivered a couple of small fixes for internal scripts.
- We've addressed an issue that made the .csv data export fail, due to line breaks added within the 'Remark' field of queries.
- We've addressed a defect on the copy form functionality regarding errors shown on the variable name.
- We have addressed an issue where, due to an underlying operating system issue, an 'Euro' sign added a field label would not be correctly exported.
Maintenance release 2022.5.2.0 - Release date 18th January 2023
System improvements: Export
For fields that span multiple columns (Geolocation, Grid, Number & Date, and Checkbox fields), we now add all the column names as rows to the variable list files.
Since we added these variables to the files, we have added two new columns to the variable list files as well. Instead of the “Field type” column, the files now contain a "Original field type" (e.g., Number & Date) and "Exported field type" (e.g., Number for row 1 and Date for row 2).
System defects fixes
We have addressed a defect that was not updating the pending SDV listing after archiving repeating data instances. Now, data points of archived instances do not show up any longer on this global verifications report.
We've fixed a defect that was causing an error when trying to create a linked study with Form Sync for studies with thousands of fields.
We fixed a defect related to "Field Created" Audit Trail events being created during Form Sync. These events should have been created to the Audit Trail of the production study, but were added to the test study's Audit Trail instead. We have also migrated the "Field Created" Audit Trail events from the (incorrect) test study to the production study.
Another issue on Form sync has been addressed. The error ‘Something went wrong’ is not longer occurring when trying to merge the changes from test to production.
We have fixed a defect related to printing eCRFs: previously fields that were supposed to be hidden due to dependency rules were still visible in the print version of an eCRF. This is now fixed.
A defect occurring on studies with GCP enabled that manifested itself on specific automations to show/hide CRF elements has been fixed. Now all of these automations are triggered as expected, according to the defined conditions.
We've fixed an internal technical issue that was sometimes causing an error to be thrown when the participant ID was being stored in the database.
We have fixed a defect regarding the numbering of fields when a CRF is being printed.
We have fixed a defect related to an error message being displayed when copying fields to other forms.
Maintenance release 2022.5.3.0 - Release date 1st February 2023
New features and / or enhancements
For a while, it has become mandatory to have unique names for your options groups. For running studies that did not yet get to amend this, we have added an error message when attempting to create a linked study using Form Sync when this situation occurs, to clarify what exactly the issue blocking the form sync process is. The copy of the error contains the actual option groups name that was found to have at least one duplicate, so you can go directly and search for it in your structure.
System defects fixes
We have resolved an issue when survey packages were created but not scheduled for a specific date and time, viewed by a clinician, then subsequently sent for completion by a participant, the listed survey package status was incorrectly listed as 'Sent' without updating.
We have updated encrypted fields to display an empty value where no response has been provided, replacing previous messages that obscured the field. No changes have been made to display when a value is present.
We have resolved an issue where printing of surveys was ignoring numbered ordering.
We have resolved an issue where the printed export of a visit form would not display previously populated grid-dropdown responses.
-
An issue was identified where it was not possible for a survey invitation's progress to reach 100% in cases where that invitation contained a survey that had been removed from the survey package. Removed surveys will now continue to be included for already scheduled survey package invitations to allow progress to reach 100%.
In future releases, survey packages and their corresponding invitation(s) will be updated to have clearer guidance and limits on editing when survey packages have already been issued or scheduled for a participant.
.Maintenance release 2022.5.4.0 - Release date 9th February 2023
System defects fixes
- We have fixed a defect regarding the numbering of fields in surveys and repeating data when a CRF is being printed.
- We have fixed a defect in the 'Print empty CRF' modal that showed users an option to print empty repeating data instances, which did not work and should not have been possible.