Managing existing participants in eConsent
Participants within eConsent can be managed by navigating to the ‘Participants’ in your eConsent Study:
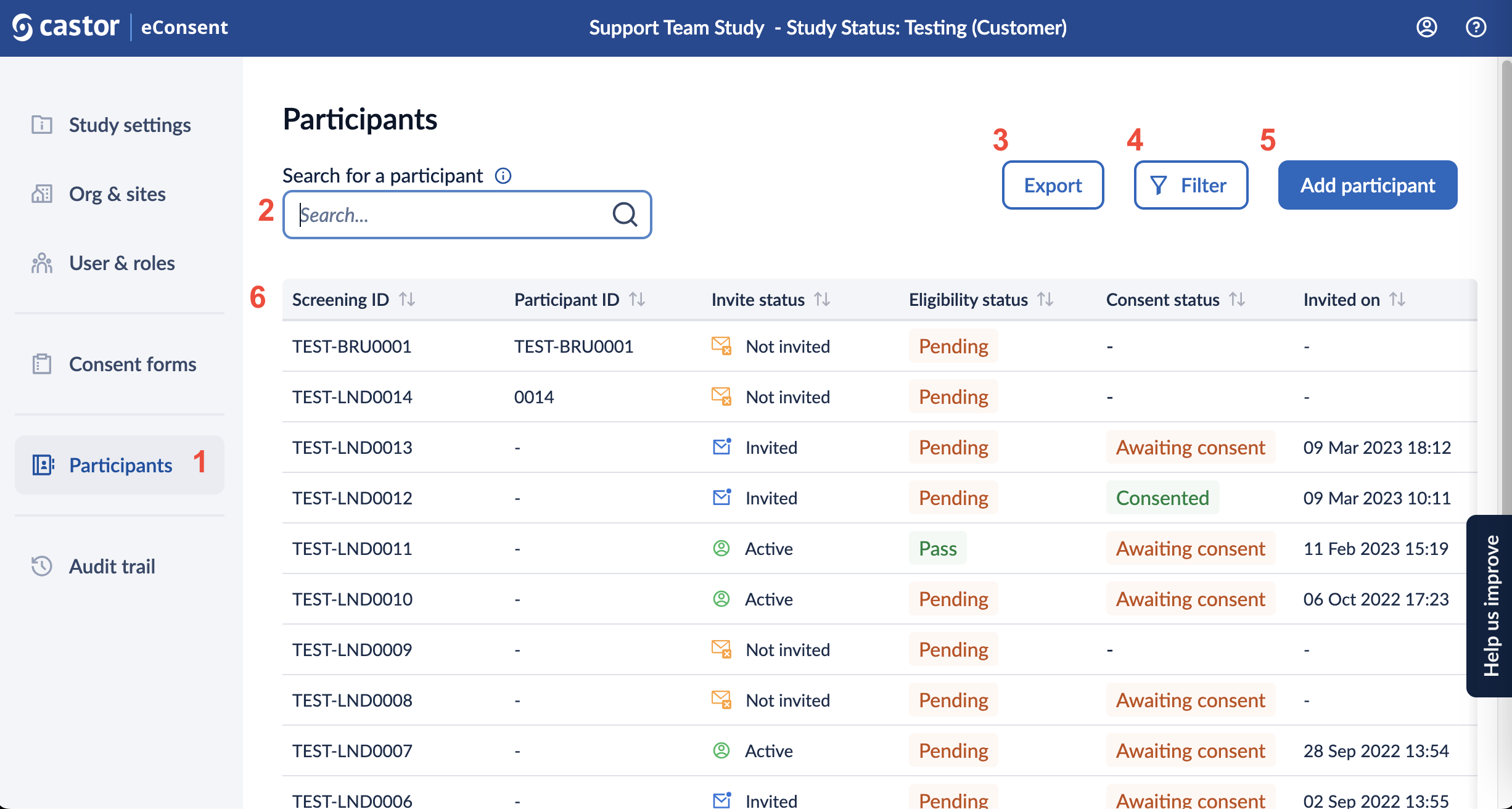
1. The ‘Participants’ tab displays a listing of all participants that you have access to, based on your role and sites.
2. Using the search bar, it is possible to search for a participant based on:
- Screening ID
- Participant ID
- Full email address, if you have access
- First or last name, if you have access
3. The export button allows exporting signature information
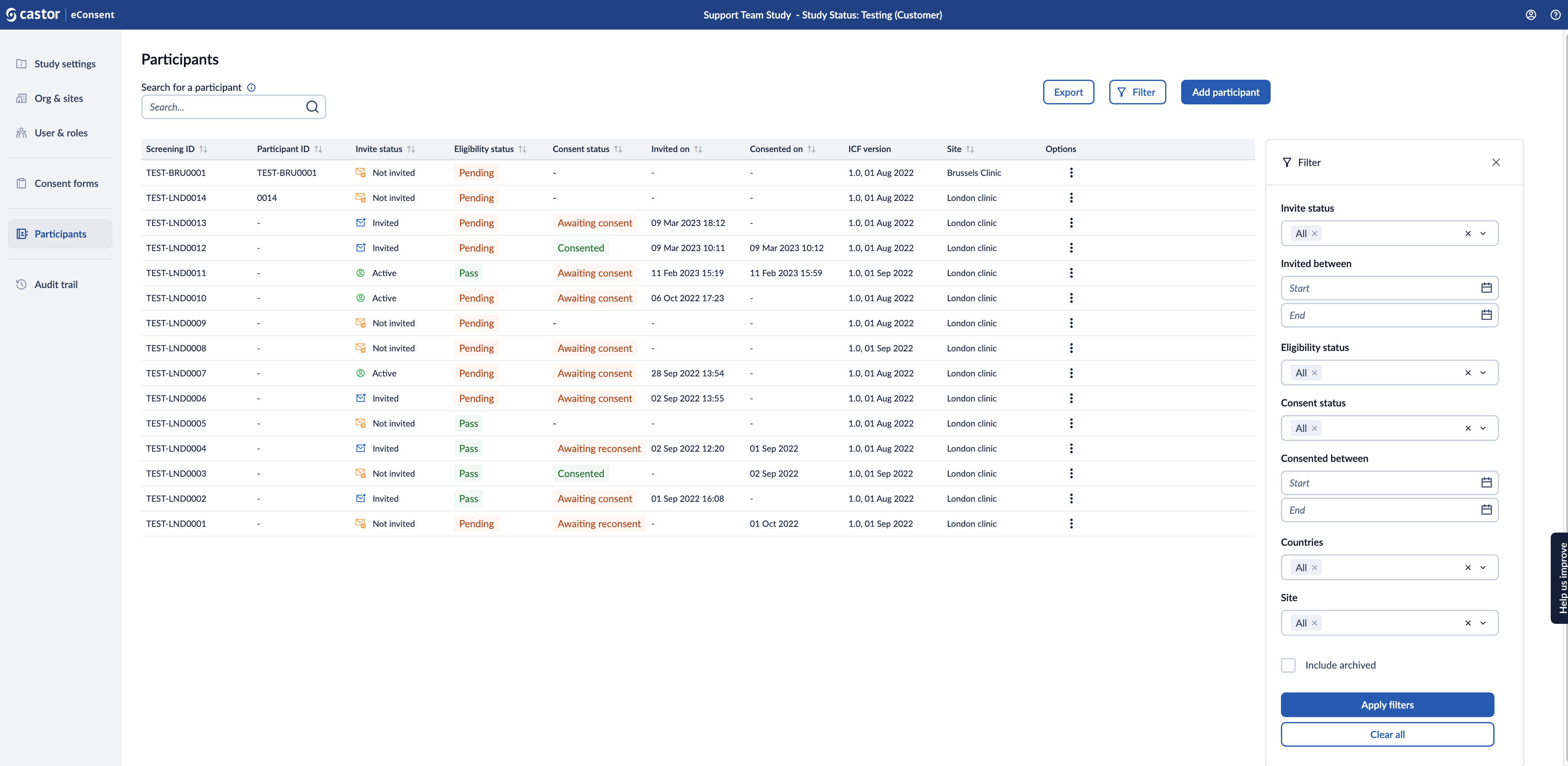
4. You can choose the ‘Filter’ option to further filter the participants based on site, invitation date range, eligibility status, consent status, consent date range, counties, and archived records. As default archived participants will not be shown in the participant overview.
5. Add participant button allows creating more participants in your study
6. The participants listing allows to view participants created in the study based on the rights assigned to you:
- screening ID: is a unique combination of characters assigned to a study participant
- participant ID: when a participant consents to joining a clinical trial, they are assigned a participant ID during the enrolment, process, the participant ID can be generated automatically or assigned manually
- invite status: indicates the status of invitations sent to participants who are being considered for enrolment in the study, the following status is available: not invited, invited, active
- eligibility status: pass, pending, ineligible. This field is used to manage and document whether individuals meet the specific criteria required to participate in the study.
- consent status: -, consented, awaiting consent, awaiting reconsent. This field reflects the status of participants' informed consent form.
- invited on: Timestamp when an email invitation has been sent to a participant.
- consented on: Timestamp when participant consented.
- ICF version: Version of the ICF.
- site: displays a specific study site or location where the participants are enrolled and data is collected.
- options: provide additional options or actions which can be performed with each participant record. When clicked , it opens a dropdown menu.
To archive a participant select the ellipsis to the right of the participant's record and select ‘archive’.
To enter the participant's profile click on the participant record. Once selected, the participant record will be opened to the profile view. You can also select ‘Consent Forms’ to see an overview of the consent forms or ‘Audit Trail’ to see information regarding the particular participant.
In the participant overview you can manage participant information by selecting the pencil icon within the corresponding locations.
In the participant Profile edit you can edit the participant's site location as well as language.
Within ‘Personal Details’ the participants first and last name, date of birth, gender, phone number, and address can be updated based on the personal details requirements set upon creation of the study.