What is the difference between the types of repeating data in EDC/CDMS?
Table of Contents
Castor CDMS allows creating two different repeating data types: measurements and unscheduled events.
Within measurements, there is only the type 'repeated measures'. Unscheduled events can be of the type: 'adverse event', 'medication', 'unscheduled visit', 'event', and 'other'.
In this article the differences between the repeated measures and other repeating data types are described.
Repeated measure
A repeated measure is as a measure, that can have more than one single value for each participant in the study, e.g. the heart rate (BPM) that needs to be measured several times throughout a study. We expect to have an undetermined number of measurements, so adding only one regular number field to a form would not be sufficient. In that case you can use a repeated measure.
Repeated measures have an additional feature that differentiates them from the other repeating data. They can be added to any step as a field and the repeated results will be visualized in the form:
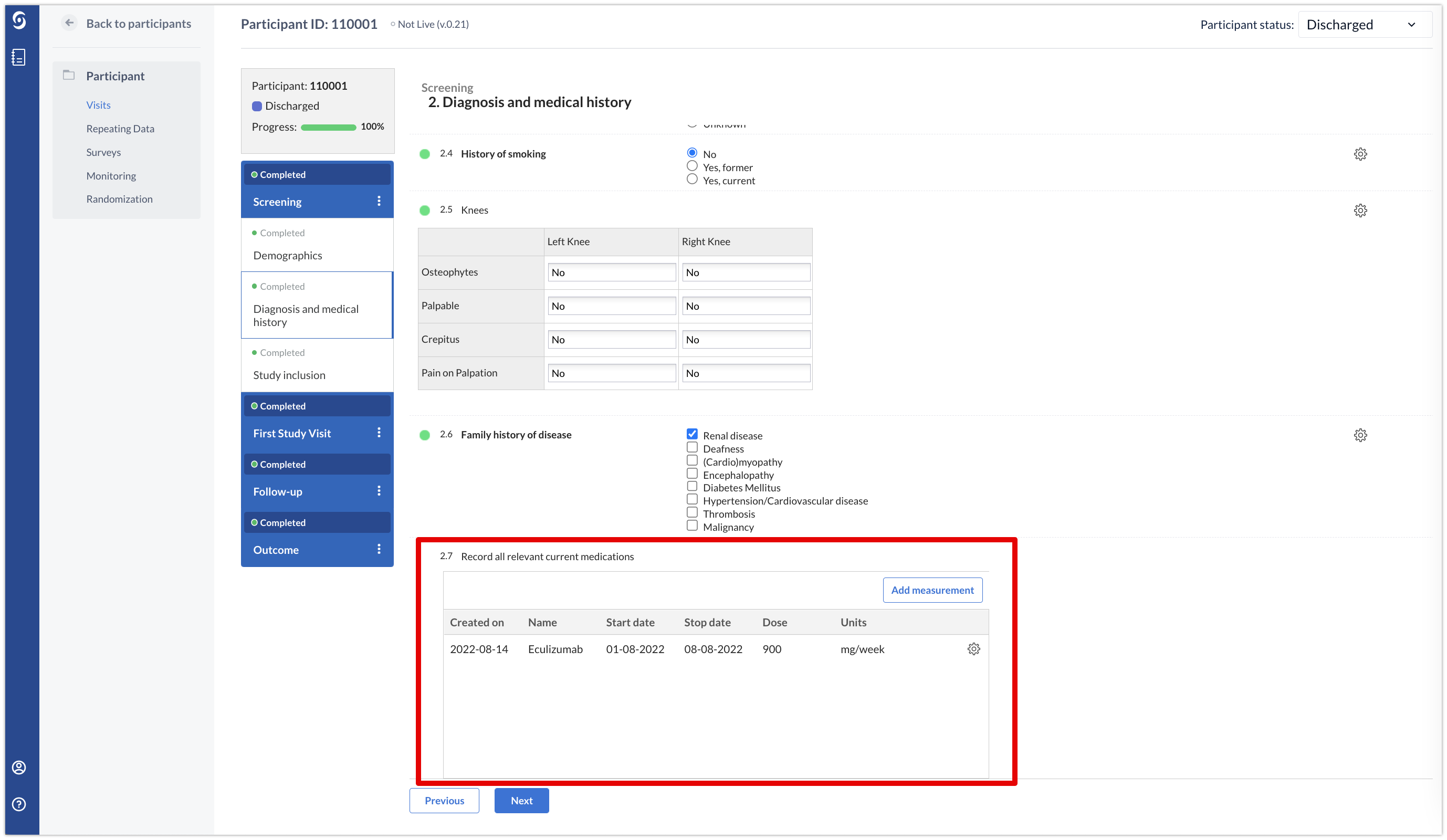
A repeated measure can only consist of one form.
See also the differences and similarities between repeated measures and grid fields.
Unscheduled repeating data types
The other repeating data form types fall under one category, the unscheduled repeating data. For these repeating data types, it is possible to add a 'Repeating Data' button to your study forms. Other than the naming convention, there are no functional differences between these repeating data types.
Adverse Event (AE)
Register here any unexpected and undesirable experience occurring in relation to the use of medication. An AE will be considered SAE (Serious Adverse Event) if it results in: hospitalization, congenital abnormality, permanent disability or damage, life-threatening conditions, or death.
Medication
This repeating data is used to keep track of other concomitant medications, that is not directly related to the study itself.
Unscheduled visit
The data gathered in an ‘unscheduled visit’ repeating data depends greatly on your study. Sometimes information is missing from a previous visit and the patient is interviewed again. It can also be that the patient requests a new visit to confirm the treatment is going as expected, if there are potential adverse symptoms.
Event
The ‘event’ repeating data type is a broad concept to register any event that could be relevant for the outcome of the study. An example would be a patient being unavailable for some time during the study for personal reasons. It may be that a visit needs to be re-scheduled and an explanation provided via an event repeating data.
Other
The repeating data type 'other' contains information not applicable to the other kinds of repeating data. A very specific use of this kind of repeating data is detailed in the article Notifications for Serious Adverse Events.
Read more about repeating data in the articles: Create a repeating data form, Entering repeated data, and The 'Repeating Data Button' field.