Transforming a paper CRF into a Castor CDMS study structure
Table of Contents
This section will demonstrate how you can build Castor CDMS study structure from a paper case form.
This example CRF page captures Demographics data:

The Study structure tab allows you to outline the different forms, i.e. sections that contain related data.
Study forms: Visits/Forms
Visits in Castor are used to represent planned study visits. In the example below, one Visit ‘Baseline Visit’ is created. A Form is a form within this Visit that groups one or more related questions (fields). In our example, the baseline visit contains groups of questions related to ‘Demographics’, ‘Informed consent’, ‘Inclusion criteria’ and ‘Exclusion criteria’. The relevant Forms are thus created in the Baseline visit Visit:
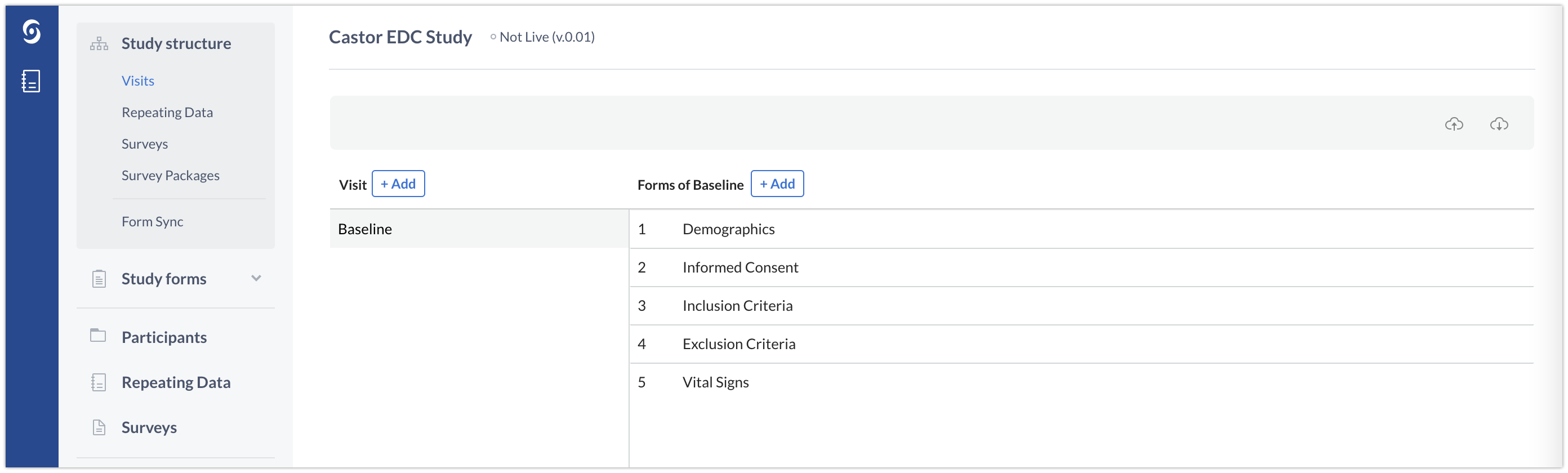
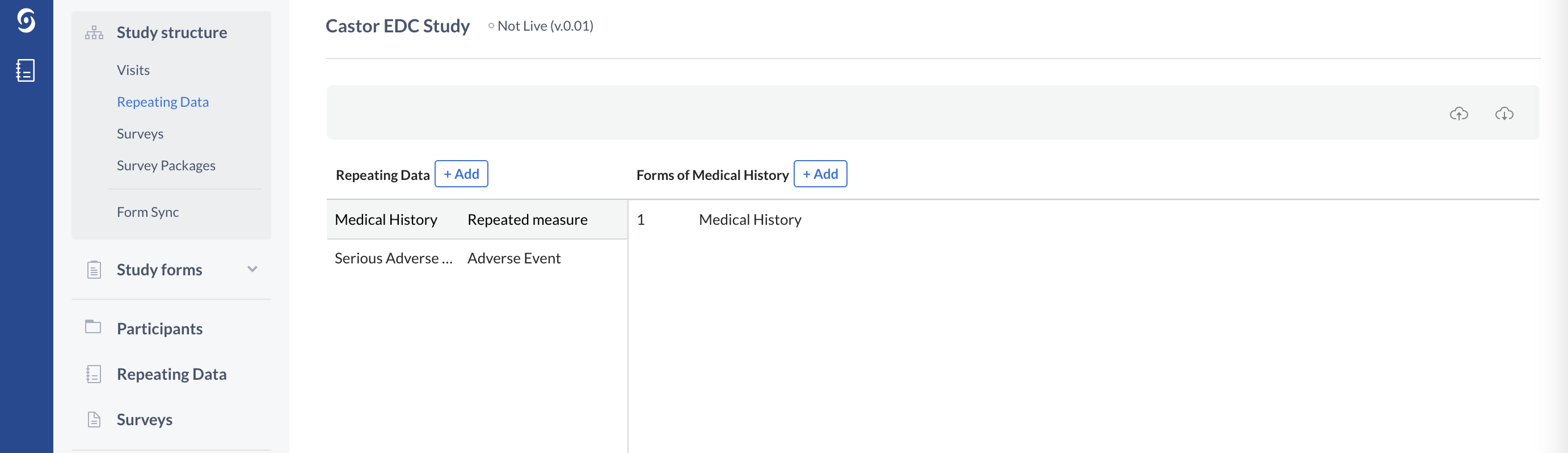
Repeating Data: Repeated Measures
Looking at the CRF example in the beginning of this article, we see a tabular representation of Medical history. This section contains information about all relevant medical conditions of a subject. One patient can have several relevant medical conditions and another patient can have none. Such instances that can occur 0-N times for each participant require a Repeating Data form in Castor. With such repetitive instances, it is often convenient to choose the Repeated Measure type of Repeating Data structure, as this can be displayed in a tabular format (see below).
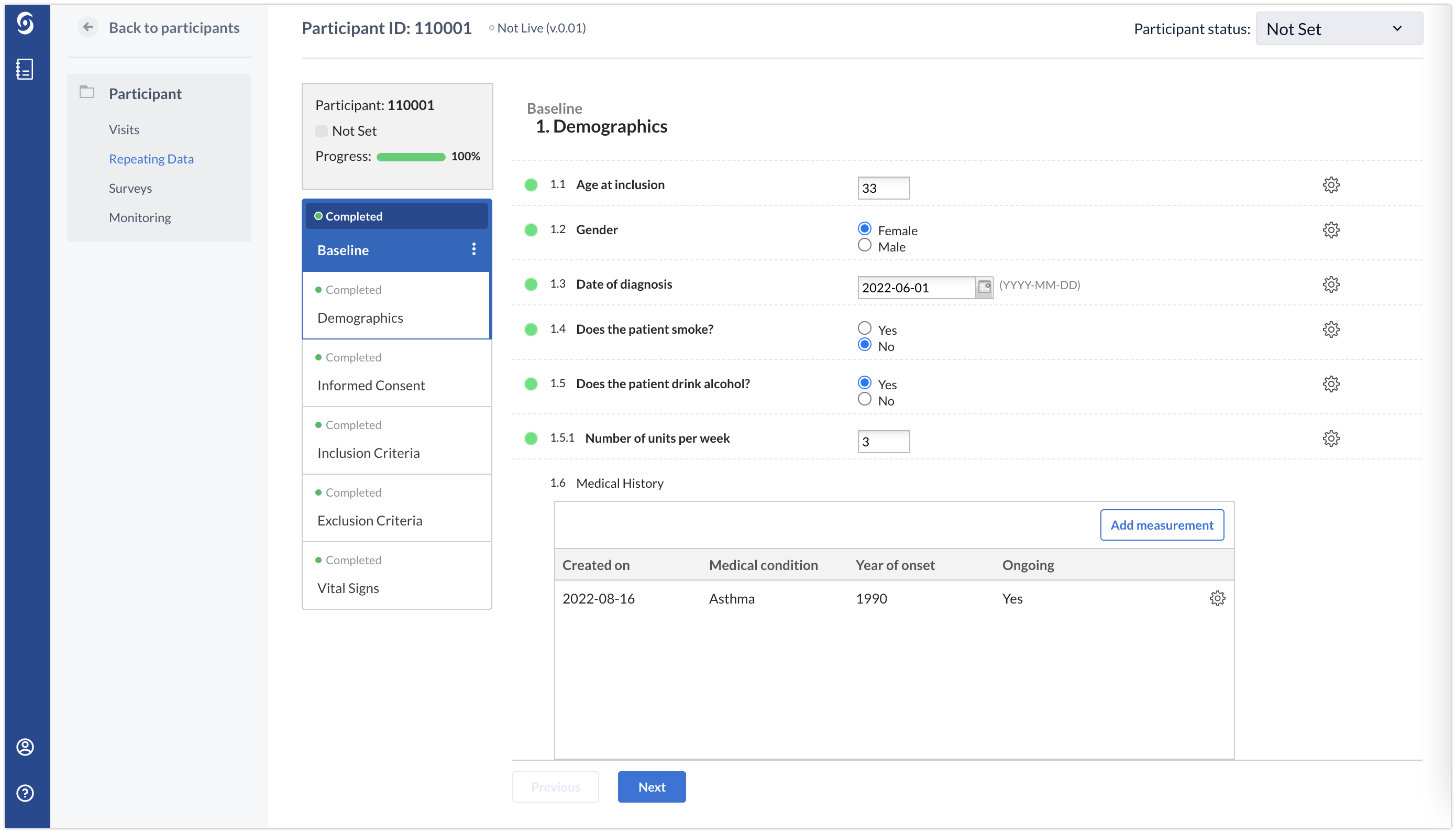
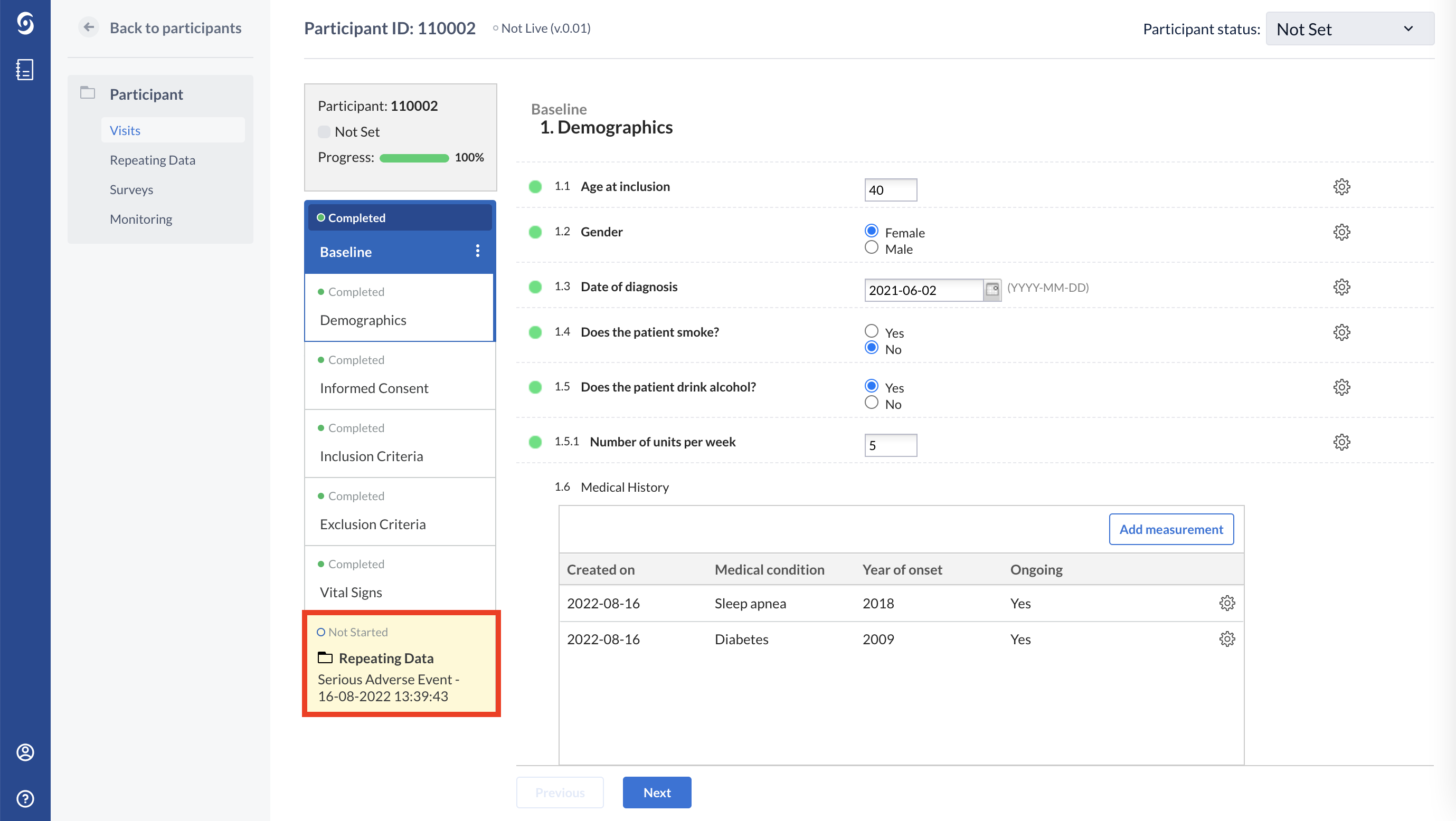
Below you will see how the Medical history repeated measure looks like in the eCRF for one participant. Using the ‘Add measurement’ button, any number of conditions can be added. In the example below, a patient has only one medical condition:
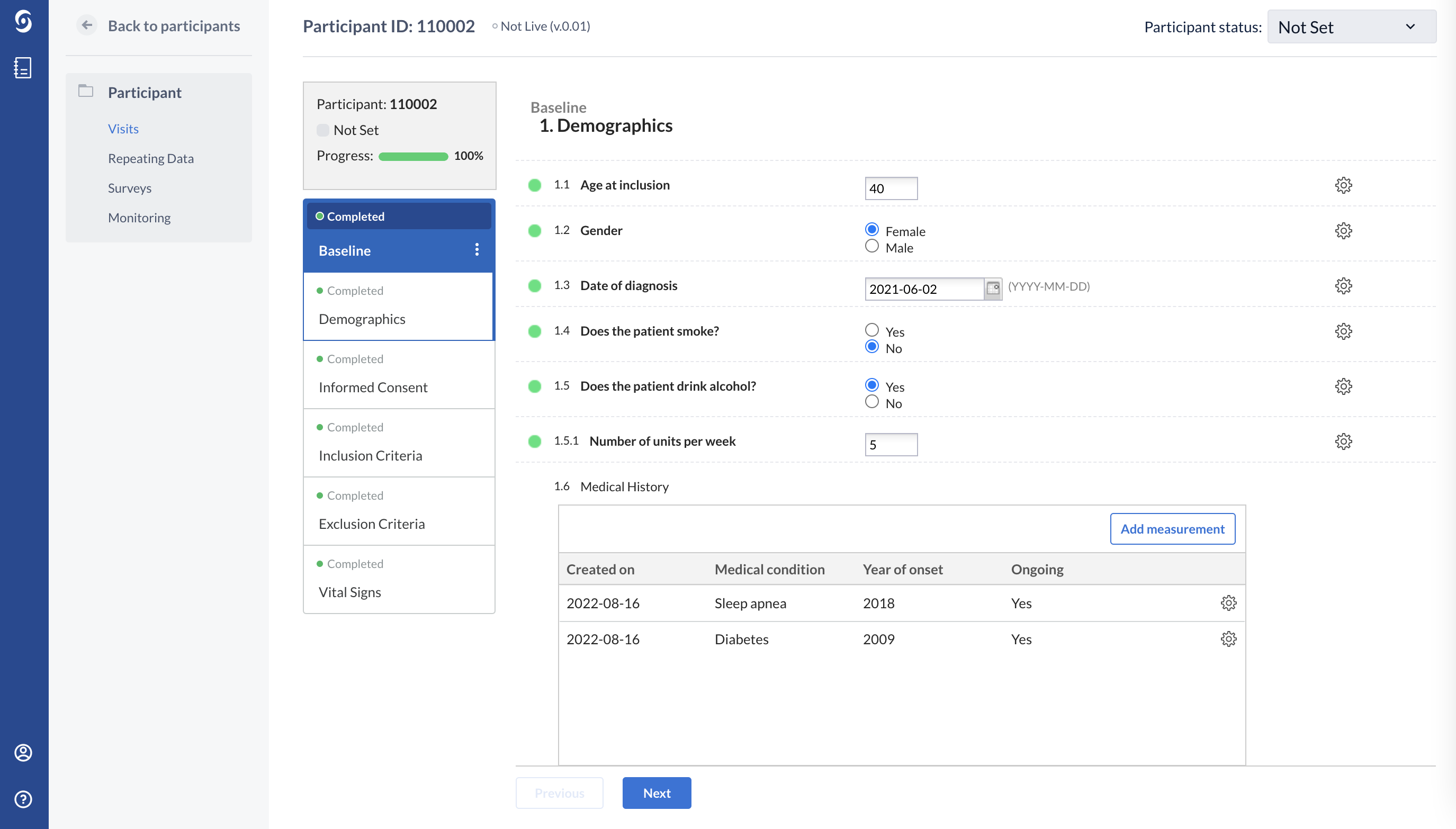
This patient has more than one medical condition:
Other common examples where a Repeated measure form can be used to collect data are Blood pressure and Concomitant medications.
Repeating Data: Serious Adverse Events and other events
Repeating Data forms should also be used in case any unintended events occur which are not part of the main study data capture. The reason for this follows the same 0-N principle as repeated measures - it is unknown how many times these events will occur for a patient, and therefore it is best to use a Repeating Data form instead of a study form. The advantage of a Repeating Data forms is that once the structure is created, a Repeating Data form can be generated any number of times and can be linked to any Visit of a study.

The Repeating Data form structure, type 'Adverse Event' in Castor:
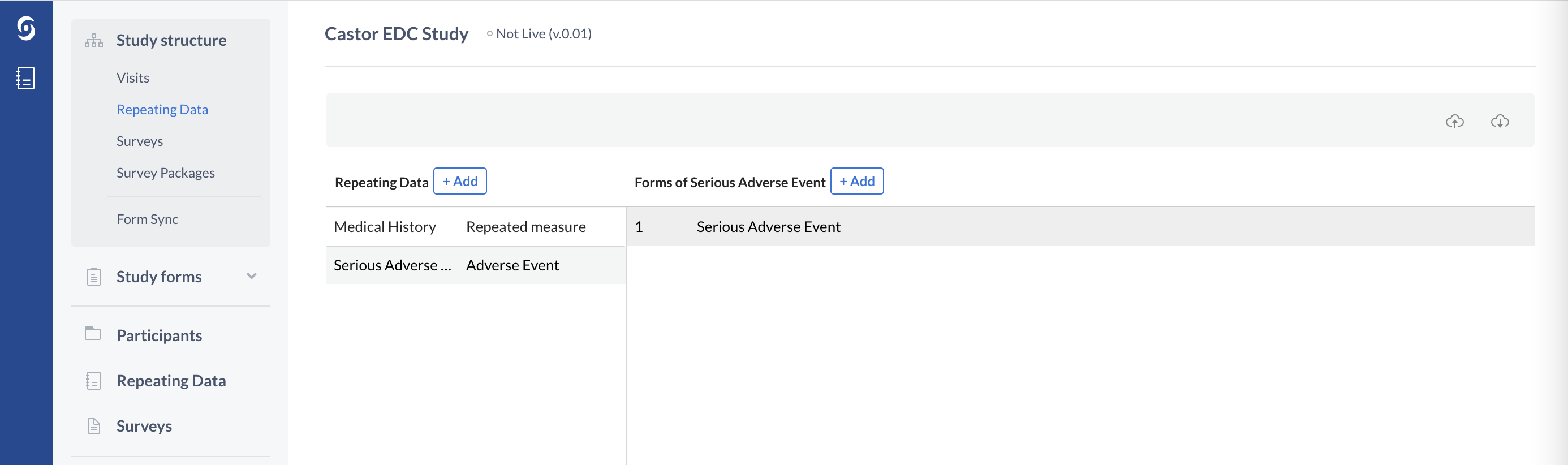
Repeating Data structures, similarly to study Visits, also consist of Forms.
In the example below one Serious Adverse Event Repeating Data form is created and linked to the Baseline Visit:
Surveys and survey packages are used when study participants are the ones entering data, for example, via a link sent electronically.
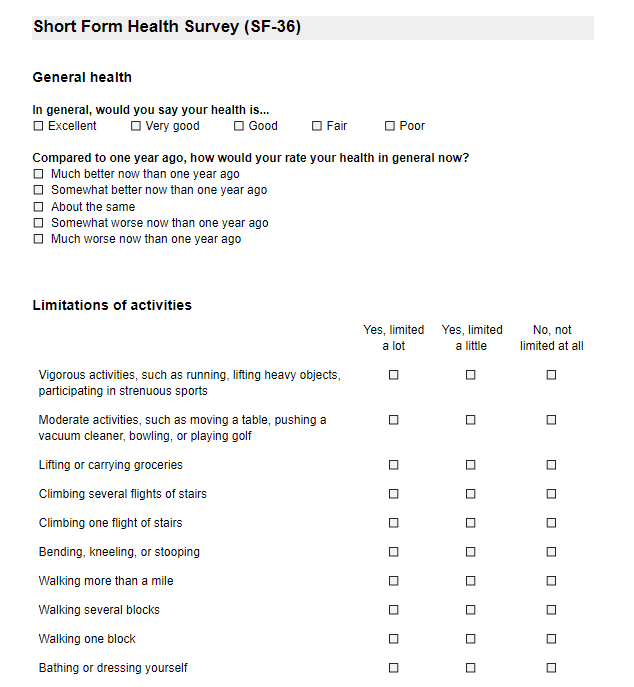
The survey structure also consists of Forms, which represent the different survey pages:
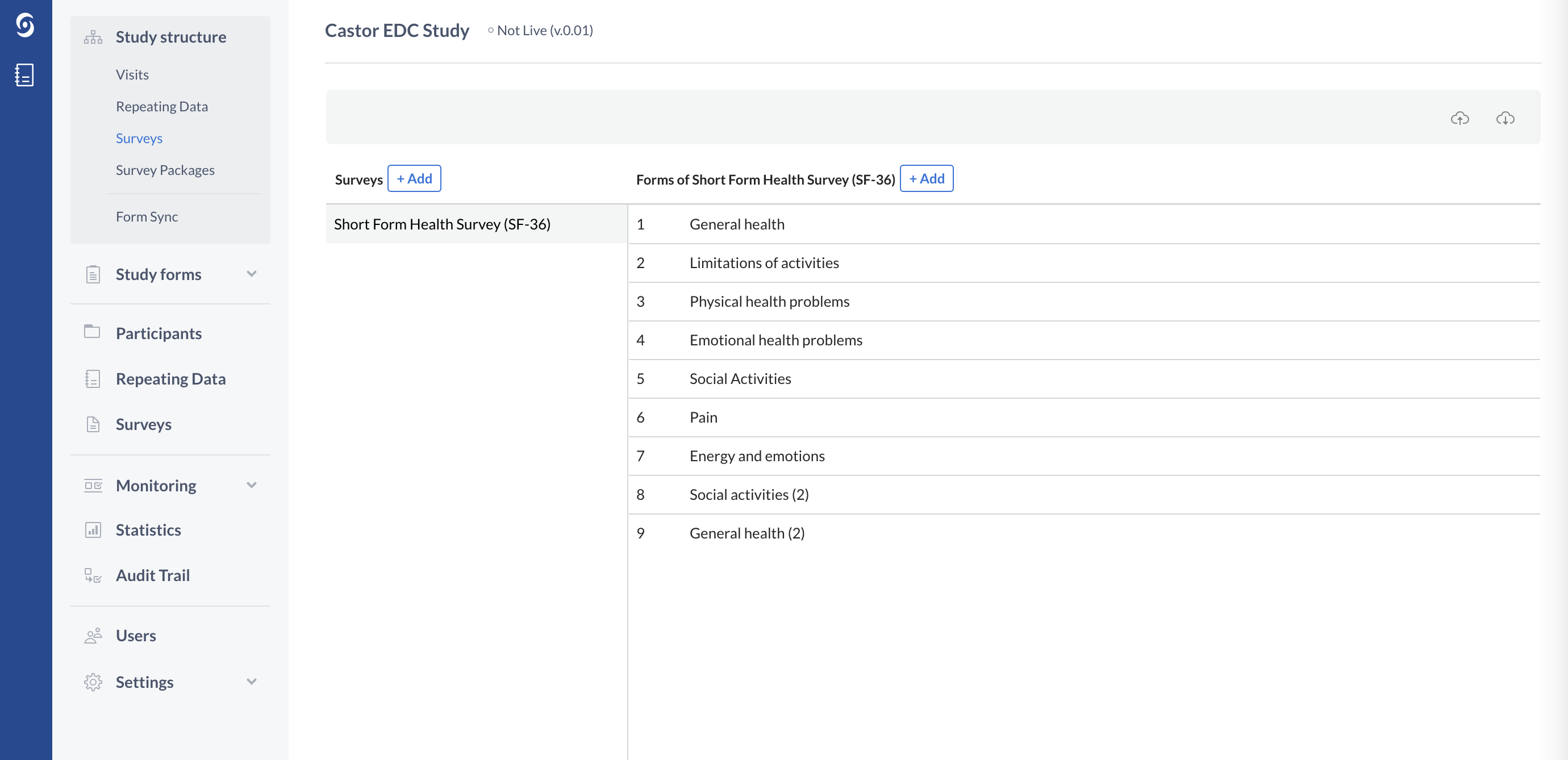
You can add questions (fields) to the survey structure via the Study forms tab.
In order to send the surveys electronically to your study participants, you will also need to create a survey package:
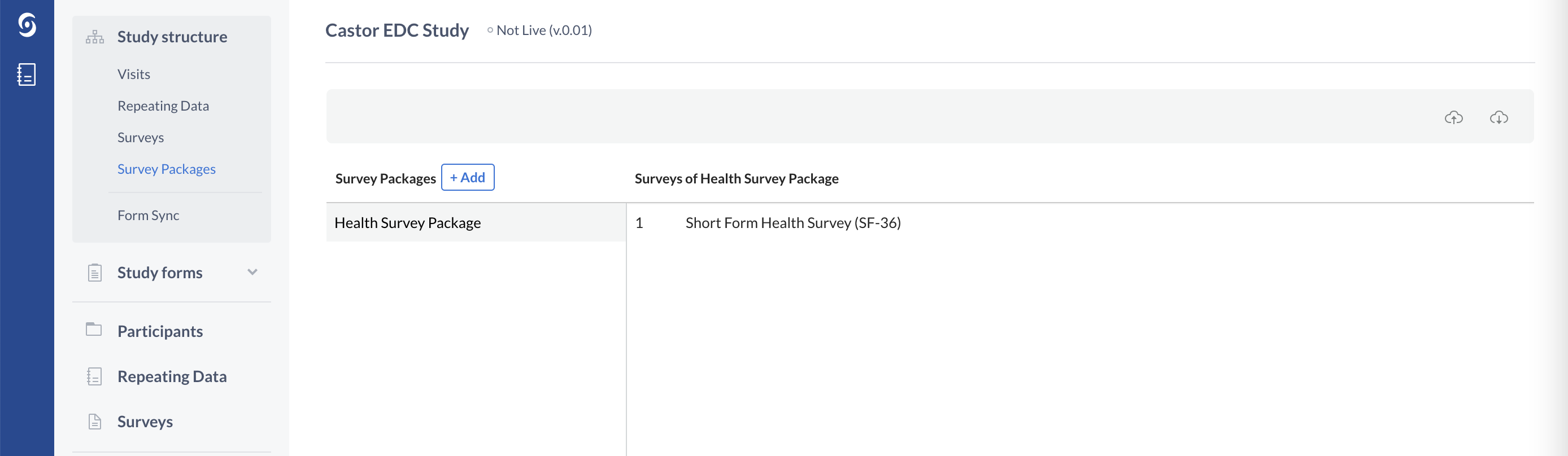
You can think of survey packages as the envelopes or ‘packages’ in which you put the surveys. You can then send this survey package to a patient via email. This email contains a link to the survey package, where the participant can immediately fill in data.